Application Of Solid-Liquid Equilibrium . The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The solid−liquid equilibrium of salt. In the present work, the minimization of the gibbs free energy was chosen as a.
from studylib.net
In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The solid−liquid equilibrium of salt. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions:
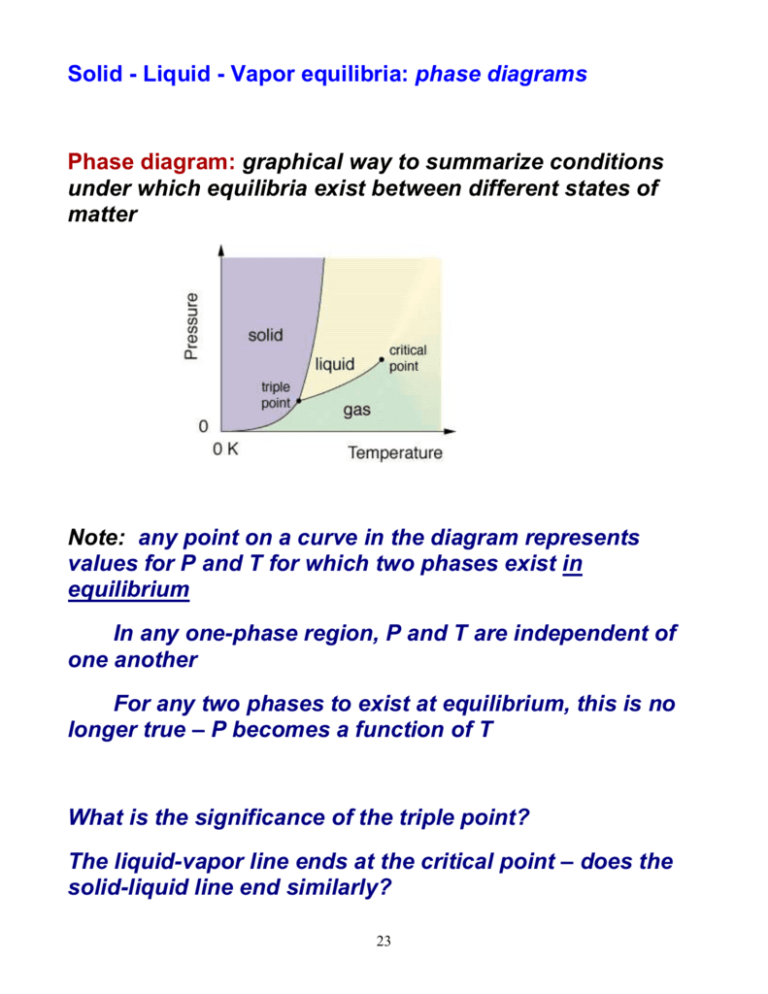
Solid Liquid Vapor equilibria phase diagrams
Application Of Solid-Liquid Equilibrium A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt.
From www.youtube.com
SolidLiquid Equilibrium YouTube Application Of Solid-Liquid Equilibrium Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to. Application Of Solid-Liquid Equilibrium.
From www.slideserve.com
PPT States of Matter and Intermolecular Forces PowerPoint Application Of Solid-Liquid Equilibrium A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In. Application Of Solid-Liquid Equilibrium.
From www.mdpi.com
Molecules Free FullText Modeling of SolidLiquid Equilibria in Application Of Solid-Liquid Equilibrium Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. In. Application Of Solid-Liquid Equilibrium.
From www.researchgate.net
Liquidvapor equilibrium of ethanolwater mixture with example Application Of Solid-Liquid Equilibrium The solid−liquid equilibrium of salt. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In. Application Of Solid-Liquid Equilibrium.
From slideplayer.com
Phase Changes. ppt download Application Of Solid-Liquid Equilibrium The solid−liquid equilibrium of salt. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In the present work, the minimization of the gibbs free energy was chosen as a. The thermodynamics of. Application Of Solid-Liquid Equilibrium.
From ivypanda.com
SolidLiquid Equilibrium in Binary System Test 1922 Words Essay Example Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The solid−liquid equilibrium of salt. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. Consider an equilibrium between a crystalline. Application Of Solid-Liquid Equilibrium.
From studycorgi.com
SolidLiquid Equilibrium in a Binary System Free Essay Example Application Of Solid-Liquid Equilibrium The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The solid−liquid equilibrium of salt. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. In. Application Of Solid-Liquid Equilibrium.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? Application Of Solid-Liquid Equilibrium The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In. Application Of Solid-Liquid Equilibrium.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Application Of Solid-Liquid Equilibrium A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. In. Application Of Solid-Liquid Equilibrium.
From www.showme.com
Liquid Vapor Equilibrium Science, Chemistry, Gases ShowMe Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of solid−liquid equilibrium is applied to. Application Of Solid-Liquid Equilibrium.
From regina-yersblogcrane.blogspot.com
Dynamic Equilibrium Between Liquid and Vapor Is Established When Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. Consider an equilibrium between a crystalline. Application Of Solid-Liquid Equilibrium.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Application Of Solid-Liquid Equilibrium Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. In. Application Of Solid-Liquid Equilibrium.
From www.researchgate.net
Phase diagram of solid liquid equilibrium in the system H 2 O FeSO Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of. Application Of Solid-Liquid Equilibrium.
From fyonszubj.blob.core.windows.net
Solid Liquid Equilibrium at Thomas McCann blog Application Of Solid-Liquid Equilibrium The solid−liquid equilibrium of salt. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. In the present work, the minimization of the gibbs free energy was chosen as a. Consider an equilibrium between a crystalline. Application Of Solid-Liquid Equilibrium.
From dokumen.tips
(PDF) MODELING AND SIMULATION OF SOLIDLIQUID EQUILIBRIUM BY … · 2018 Application Of Solid-Liquid Equilibrium Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The solid−liquid equilibrium of salt. In. Application Of Solid-Liquid Equilibrium.
From socratic.org
What do we mean by a dynamic equilibrium? Can you describe how the Application Of Solid-Liquid Equilibrium Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The solid−liquid equilibrium of salt. The thermodynamics of. Application Of Solid-Liquid Equilibrium.
From www.researchgate.net
A Pressure/Temperature equilibrium phase diagram of a one component Application Of Solid-Liquid Equilibrium Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. In the present work, the minimization. Application Of Solid-Liquid Equilibrium.
From byjus.com
Equilibrium Involving Dissolution Of Solid Gas In Liquid Henry's Law Application Of Solid-Liquid Equilibrium A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The solid−liquid equilibrium of salt. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In the present work, the minimization of the gibbs free energy was chosen as a. The thermodynamics of. Application Of Solid-Liquid Equilibrium.
From www.researchgate.net
Solidliquid equilibrium diagram of therapeutic eutectic building Application Of Solid-Liquid Equilibrium Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The solid−liquid equilibrium of salt. In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of. Application Of Solid-Liquid Equilibrium.
From www.researchgate.net
Simple Binary SolidLiquid Equilibrium Diagram Download Scientific Application Of Solid-Liquid Equilibrium A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. In the present work, the minimization of the gibbs free energy was chosen as a. The solid−liquid equilibrium of salt. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of. Application Of Solid-Liquid Equilibrium.
From www.researchgate.net
Equilibrium coexistence of ice and water. Illustration of potential Application Of Solid-Liquid Equilibrium The solid−liquid equilibrium of salt. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus. Application Of Solid-Liquid Equilibrium.
From present5.com
Chapter 3 Chemical Equilibrium Reactions Irreversible Reversible The Application Of Solid-Liquid Equilibrium The solid−liquid equilibrium of salt. In the present work, the minimization of the gibbs free energy was chosen as a. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. A new apparatus. Application Of Solid-Liquid Equilibrium.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. A new apparatus. Application Of Solid-Liquid Equilibrium.
From www.snexplores.org
Explainer What are the different states of matter? Application Of Solid-Liquid Equilibrium A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In the present work, the minimization of the gibbs free energy was chosen as a. The thermodynamics of solid−liquid equilibrium is applied to. Application Of Solid-Liquid Equilibrium.
From www.slideserve.com
PPT 17 Equilibrium (AHL) PowerPoint Presentation, free download ID Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. Consider an equilibrium between a crystalline. Application Of Solid-Liquid Equilibrium.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Application Of Solid-Liquid Equilibrium The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. In the present work, the minimization of the gibbs free energy was chosen as a. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: The solid−liquid equilibrium of salt. A new apparatus. Application Of Solid-Liquid Equilibrium.
From www.researchgate.net
(PDF) Application of PengRobinson Equation of State for Calculating Application Of Solid-Liquid Equilibrium The solid−liquid equilibrium of salt. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus. Application Of Solid-Liquid Equilibrium.
From kunduz.com
[ANSWERED] Select the incorrect statement For solid liquid equilibrium Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The solid−liquid equilibrium of salt. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. Consider an equilibrium between a crystalline. Application Of Solid-Liquid Equilibrium.
From newlasertagatlanta.blogspot.com
39 solid liquid phase diagram Wiring Diagrams Manual Application Of Solid-Liquid Equilibrium The solid−liquid equilibrium of salt. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. In the present work, the minimization of the gibbs free energy was chosen as a. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. Consider an equilibrium between a crystalline. Application Of Solid-Liquid Equilibrium.
From www.researchgate.net
Phase diagram and the phase equilibrium line 1 solid and liquid Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The solid−liquid equilibrium of salt. Consider an equilibrium between a crystalline. Application Of Solid-Liquid Equilibrium.
From www.slideserve.com
PPT SolidLiquid Extraction Solid liquid extraction(leaching) means Application Of Solid-Liquid Equilibrium The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. The solid−liquid equilibrium of salt. In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline. Application Of Solid-Liquid Equilibrium.
From www.weltbild.de
MODELING OF SOLIDLIQUID EQUILIBRIUM Buch versandkostenfrei Weltbild.de Application Of Solid-Liquid Equilibrium A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. The solid−liquid equilibrium of salt. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In the present work, the minimization of the gibbs free energy was chosen as a. The thermodynamics of. Application Of Solid-Liquid Equilibrium.
From studylib.net
Solid Liquid Vapor equilibria phase diagrams Application Of Solid-Liquid Equilibrium The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. In the present work, the minimization of the gibbs free energy was chosen as a. A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of. Application Of Solid-Liquid Equilibrium.
From www.numerade.com
SOLVEDIn a final equilibrium expression solids and liquid have value Application Of Solid-Liquid Equilibrium A new apparatus utilizing the tyndall effect to measure the solubility of salts in water was described. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: In the present work, the minimization of the gibbs free energy was chosen as a. The thermodynamics of solid−liquid equilibrium is applied to. Application Of Solid-Liquid Equilibrium.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Application Of Solid-Liquid Equilibrium In the present work, the minimization of the gibbs free energy was chosen as a. The solid−liquid equilibrium of salt. The thermodynamics of solid−liquid equilibrium is applied to binary systems including a semicrystalline polymer and a solvent. Consider an equilibrium between a crystalline salt (or other kind of ionic solid) and a solution containing the solvated ions: A new apparatus. Application Of Solid-Liquid Equilibrium.