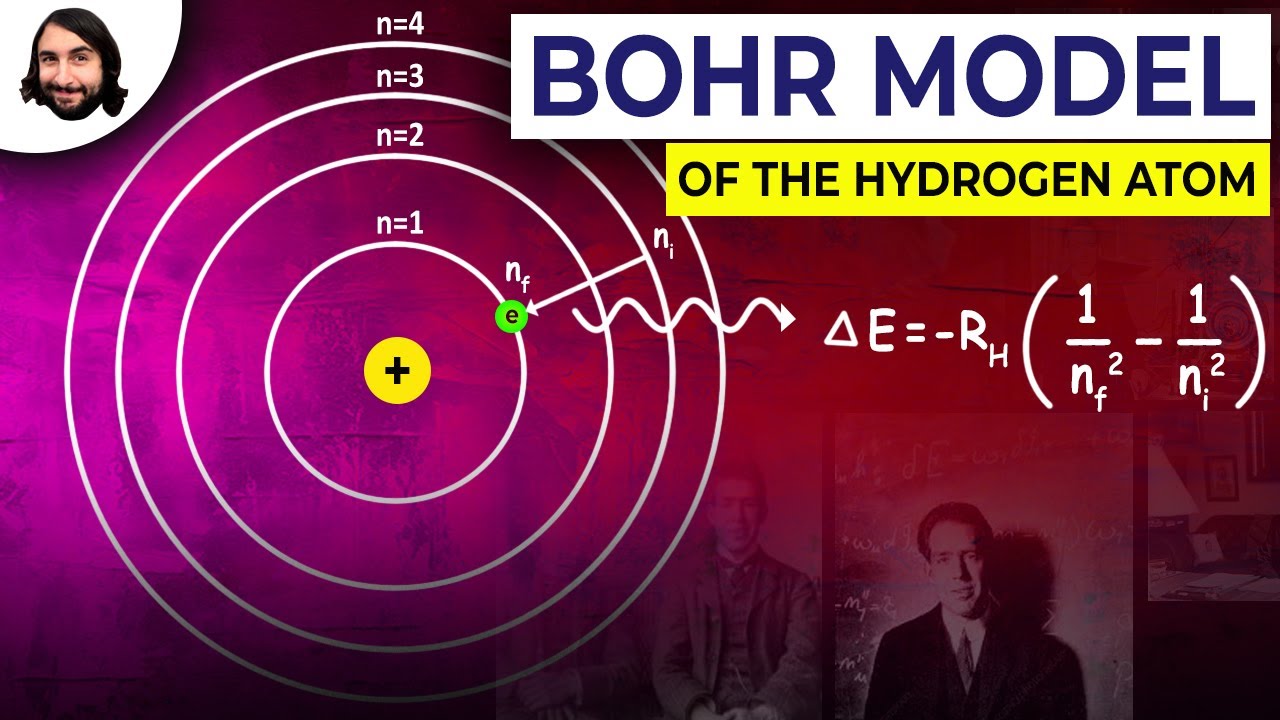
Change In Energy Level Equation . Δ e = change in energy level (j) Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. Using the properties of debroglie waves, we can. The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: The energy of light at a. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron.
from www.youtube.com
Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. The energy of light at a. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Δ e = change in energy level (j) The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Using the properties of debroglie waves, we can.
Bohr Model of the Hydrogen Atom YouTube
Change In Energy Level Equation Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Using the properties of debroglie waves, we can. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. The energy of light at a. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Δ e = change in energy level (j) The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps Change In Energy Level Equation Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. The energy of light at a. Bohr calculated the energy of an electron in the nth level of the hydrogen atom by. Change In Energy Level Equation.
From www.tessshebaylo.com
Quantum Mechanics Energy Levels Equation Tessshebaylo Change In Energy Level Equation Using the properties of debroglie waves, we can. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Bohr calculated the energy of an electron in the nth level of the hydrogen atom by. Change In Energy Level Equation.
From www.youtube.com
Calculating free energy change YouTube Change In Energy Level Equation The energy of light at a. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Usually it is emitted as light of a given wavelength. Change In Energy Level Equation.
From www.expii.com
Energy Diagram — Overview & Parts Expii Change In Energy Level Equation Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released. Change In Energy Level Equation.
From www.reddit.com
Can someone help me understand energy transitions? AAMC FL4 28 CP r/Mcat Change In Energy Level Equation Δ e = change in energy level (j) Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Using the properties of debroglie waves, we can. Each emitted photon has a. Change In Energy Level Equation.
From sciencetallis.weebly.com
1. Energy THOMAS TALLIS SCIENCE Change In Energy Level Equation Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron.. Change In Energy Level Equation.
From recipepes.com
bohr model equation Change In Energy Level Equation In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Δ e = change in energy level (j) The energy of light at a. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. The energy of. Change In Energy Level Equation.
From www.youtube.com
Calculating changes in internal energy from equations of state YouTube Change In Energy Level Equation Using the properties of debroglie waves, we can. Δ e = change in energy level (j) Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. The energy of light at a.. Change In Energy Level Equation.
From powerpointban.web.fc2.com
What is the Rydberg equation? Change In Energy Level Equation Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Usually it. Change In Energy Level Equation.
From studylib.net
Heat Equation Change In Energy Level Equation Δ e = change in energy level (j) Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Bohr calculated the energy of an electron in the nth level of the hydrogen atom by. Change In Energy Level Equation.
From www.tessshebaylo.com
Equation For Light Energy Tessshebaylo Change In Energy Level Equation Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Δ e = change in energy level (j) Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: In this explainer, we will learn how to calculate the energy. Change In Energy Level Equation.
From www.tessshebaylo.com
Equation For Energy Transferred Specific Heat Capacity Tessshebaylo Change In Energy Level Equation The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. Using the properties of debroglie waves, we can. Δ e = change in energy level (j) The energy. Change In Energy Level Equation.
From www.youtube.com
Calculate the Energy, frequency & wavelength of an electron transition Change In Energy Level Equation The energy of light at a. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Using the properties of debroglie waves, we can. Bohr calculated the energy of an electron in the nth. Change In Energy Level Equation.
From www.slideserve.com
PPT Lecture 15 Bohr Model of the Atom PowerPoint Presentation, free Change In Energy Level Equation Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. The energy of light at a. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Δ e = change in energy level (j) Using the properties of debroglie. Change In Energy Level Equation.
From www.slideserve.com
PPT ENTC 303 Announcements PowerPoint Presentation, free download Change In Energy Level Equation The energy of light at a. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: In this explainer, we will learn how to calculate the energy of the photon that is absorbed or. Change In Energy Level Equation.
From www.tessshebaylo.com
What Is The Equation Of Energy A Photon Tessshebaylo Change In Energy Level Equation Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: The energy of light at a. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. In this explainer, we will learn how to calculate the energy of the photon. Change In Energy Level Equation.
From www.adda247.com
Energy formula in Physics & Equation for Class 10, 11 and 12 Change In Energy Level Equation Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when. Change In Energy Level Equation.
From general.chemistrysteps.com
Calculating The Energy of a Photon Chemistry Steps Change In Energy Level Equation Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the. Change In Energy Level Equation.
From www.youtube.com
Using the Rydberg Equation to Solve Transitions Between Energy Levels Change In Energy Level Equation The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Using the properties of debroglie waves, we can. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. Each emitted photon has a wavelength which is associated with. Change In Energy Level Equation.
From www.slideserve.com
PPT Chapter 8 The Quantum Mechanical Atom PowerPoint Presentation Change In Energy Level Equation In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of. Change In Energy Level Equation.
From www.sliderbase.com
Electrons and WaveParticle Duality Presentation Chemistry Change In Energy Level Equation Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Δ e. Change In Energy Level Equation.
From www.youtube.com
Bond Energy Calculations & Enthalpy Change Problems, Basic Introduction Change In Energy Level Equation Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. Using the properties of debroglie waves, we can. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Johan rydberg use balmers work to derived an equation. Change In Energy Level Equation.
From www.slideserve.com
PPT Lecture 16 Bohr Model of the Atom PowerPoint Presentation, free Change In Energy Level Equation In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Δ e = change in energy level (j) Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Each emitted photon has a wavelength which is. Change In Energy Level Equation.
From misschen.com.sg
energychanges Change In Energy Level Equation The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Each. Change In Energy Level Equation.
From www.youtube.com
Example Using specific heat to calculate ideal gas internal energy Change In Energy Level Equation Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of. Change In Energy Level Equation.
From www.tessshebaylo.com
What Is The Equation Of Energy A Photon Tessshebaylo Change In Energy Level Equation In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized. Usually it is emitted as light of a given wavelength which corresponds directly to the change. Change In Energy Level Equation.
From www.slideserve.com
PPT Chapter 7 Quantum Theory of the Atom PowerPoint Presentation Change In Energy Level Equation Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. The energy of. Change In Energy Level Equation.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Change In Energy Level Equation Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: The energy of light at a. The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\). Change In Energy Level Equation.
From www.slideshare.net
Photon and energy levels Change In Energy Level Equation Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: Δ e = change in energy level (j) The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Bohr calculated the energy of an electron in the nth level of. Change In Energy Level Equation.
From www.youtube.com
How to Calculate the Energy Released During an Energy Level Transition Change In Energy Level Equation The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Bohr calculated the energy of an electron in the nth level of the hydrogen atom by considering the electrons to be quantized.. Change In Energy Level Equation.
From www.shutterstock.com
Change Gravitational Potential Energy Formula Scientific Stock Vector Change In Energy Level Equation Δ e = change in energy level (j) In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. Using the properties of debroglie waves, we can. The. Change In Energy Level Equation.
From online-learning-college.com
Energy level diagrams Endothermic & Exothermic reactions Change In Energy Level Equation In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. The energy of the photon \(e\) absorbed/released during the transition is equal to the energy change \(\delta e\) of the electron. The energy of light at a. Each emitted photon has a wavelength which is associated with a. Change In Energy Level Equation.
From lesmylscuisine.blogspot.com
Standard Gibbs Free Energy Equation Lesmyl Scuisine Change In Energy Level Equation Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy of the electron.. Change In Energy Level Equation.
From www.youtube.com
Energy level diagram of an electron in the Hydrogen atom part2 YouTube Change In Energy Level Equation The energy of light at a. Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. In this explainer, we will learn how to calculate the energy of the photon that is absorbed or released when an electron. The energy of the photon \(e\) absorbed/released during the transition is equal to the energy. Change In Energy Level Equation.
From www.youtube.com
Bohr Model of the Hydrogen Atom YouTube Change In Energy Level Equation Johan rydberg use balmers work to derived an equation for all electron transitions in a hydrogen atom. Each emitted photon has a wavelength which is associated with a discrete change in energy, according to the equation: The energy of light at a. Usually it is emitted as light of a given wavelength which corresponds directly to the change in energy. Change In Energy Level Equation.