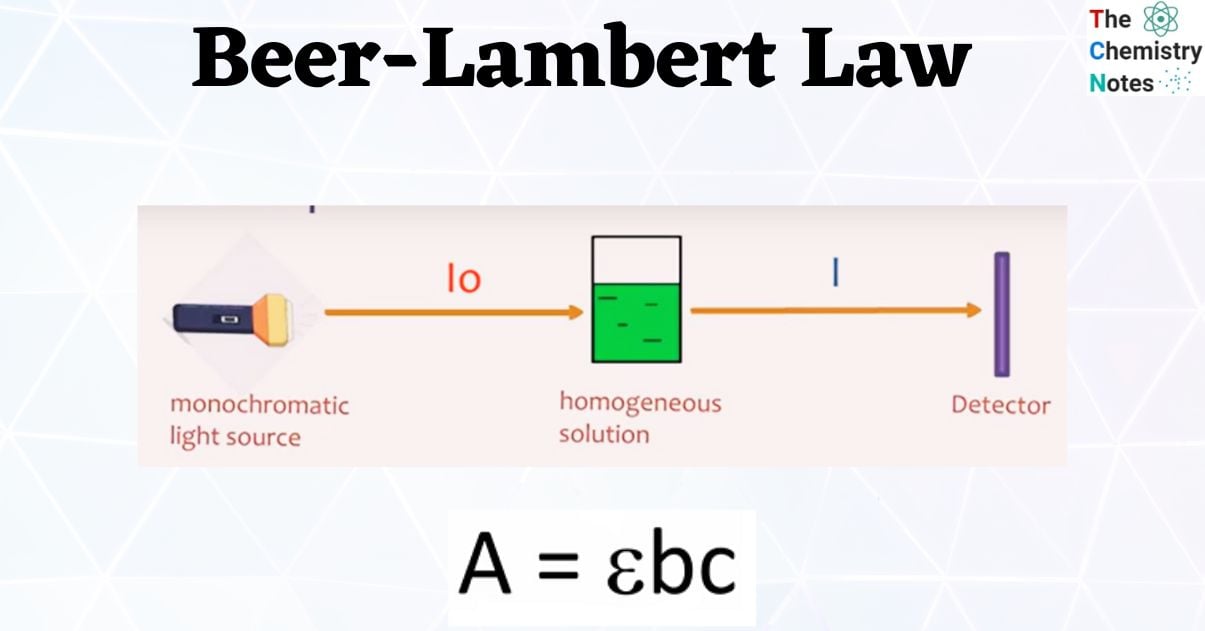
Beer's Law In Y=Mx+B Form . Once you have that you can compare the absorbance value of an. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. You can also use this calculator to determine the. A = ( l)c with the general form y =. The basic idea here is to use a graph plotting absorbance vs. The equation for beer’s law is a straight line with the general form of y = mx +b.
from scienceinfo.com
A = ( l)c with the general form y =. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The basic idea here is to use a graph plotting absorbance vs. You can also use this calculator to determine the. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The equation for beer’s law is a straight line with the general form of y = mx +b. Once you have that you can compare the absorbance value of an.
BeerLambert Law Statement, Derivation, Applications, Limitations
Beer's Law In Y=Mx+B Form Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Once you have that you can compare the absorbance value of an. The basic idea here is to use a graph plotting absorbance vs. The equation for beer’s law is a straight line with the general form of y = mx +b. A = ( l)c with the general form y =. You can also use this calculator to determine the. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions.
From www.transtutors.com
(Solved) Beer's Law States That A = Elc, Where A Is Absorbance, C Is Beer's Law In Y=Mx+B Form Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. You can also use this calculator to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Once you have that you can compare the absorbance. Beer's Law In Y=Mx+B Form.
From www.slideserve.com
PPT Y = mx + b PowerPoint Presentation, free download ID5575322 Beer's Law In Y=Mx+B Form Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Once you have that you can compare the absorbance value of an. The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =. You can also use. Beer's Law In Y=Mx+B Form.
From brainly.com
Write an equation in y = mx + b form to represent this table. Beer's Law In Y=Mx+B Form Once you have that you can compare the absorbance value of an. You can also use this calculator to determine the. The basic idea here is to use a graph plotting absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Calculate the gradient (m) for the equation. Beer's Law In Y=Mx+B Form.
From kunduz.com
[ANSWERED] Write an equation in y mx b form for the linear relationship Beer's Law In Y=Mx+B Form The basic idea here is to use a graph plotting absorbance vs. The equation for beer’s law is a straight line with the general form of y = mx +b. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. A = ( l)c with the general form. Beer's Law In Y=Mx+B Form.
From www.cuemath.com
y = mx + b What is Meaning of y = mx + b, How to Find Slope and Y Beer's Law In Y=Mx+B Form Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The basic idea here is to use a graph plotting absorbance vs. The equation for beer’s law is. Beer's Law In Y=Mx+B Form.
From sciencenotes.org
Beer's Law Equation and Example Beer's Law In Y=Mx+B Form You can also use this calculator to determine the. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The equation for beer’s law is a straight line with the general form of y = mx +b. The basic idea here is to use a graph plotting absorbance vs. A. Beer's Law In Y=Mx+B Form.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law In Y=Mx+B Form The equation for beer’s law is a straight line with the general form of y = mx +b. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions.. Beer's Law In Y=Mx+B Form.
From www.numerade.com
SOLVED In the sketch of a Beers law plot below, label the x and y axis Beer's Law In Y=Mx+B Form Once you have that you can compare the absorbance value of an. The equation for beer’s law is a straight line with the general form of y = mx +b. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Calculate the gradient (m) for the equation of the line. Beer's Law In Y=Mx+B Form.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law In Y=Mx+B Form Once you have that you can compare the absorbance value of an. The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Since the concentration, path. Beer's Law In Y=Mx+B Form.
From www.youtube.com
Slope Intercept Form Y=mx+b Algebra YouTube Beer's Law In Y=Mx+B Form The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. You can also use this calculator to determine the. Once you have that you can compare the absorbance. Beer's Law In Y=Mx+B Form.
From slideplayer.com
Beer’s Law P0 Uses of Beer’s Law ppt download Beer's Law In Y=Mx+B Form You can also use this calculator to determine the. Once you have that you can compare the absorbance value of an. The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data. Beer's Law In Y=Mx+B Form.
From www.cuemath.com
y = mx + b Beer's Law In Y=Mx+B Form Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Once you have that you can compare the absorbance value of an. The basic idea here is to use a graph plotting absorbance vs. You can also use this calculator to determine the. The equation for beer’s law is a. Beer's Law In Y=Mx+B Form.
From slideplayer.com
4.4 The SlopeIntercept Form of the Equation of a Line ppt download Beer's Law In Y=Mx+B Form Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. You can also use this calculator to determine the. The equation for beer’s law is a straight line with the general form of y = mx +b. The basic idea here is to use a graph plotting absorbance. Beer's Law In Y=Mx+B Form.
From thienvienchannguyen.net
Beer Lambert's Law, Absorbance \u0026 Transmittance Spectrophotometry Beer's Law In Y=Mx+B Form Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =. Once you have that you can compare the absorbance value of an. You can also use this calculator. Beer's Law In Y=Mx+B Form.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer's Law In Y=Mx+B Form Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The equation for beer’s law is a straight line with the general form of y = mx +b. The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =.. Beer's Law In Y=Mx+B Form.
From www.coursehero.com
[Solved] When you turn beers law into y=mx+b, what components of beers Beer's Law In Y=Mx+B Form Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =. You can also use this calculator to determine the. The equation for beer’s law is a. Beer's Law In Y=Mx+B Form.
From www.chegg.com
Solved write an equation of the form y=mx+b for the Beer's Law In Y=Mx+B Form Once you have that you can compare the absorbance value of an. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =. You can also use this calculator. Beer's Law In Y=Mx+B Form.
From www.showme.com
Using Slopeintercept form to write equations in y=mx+b Math ShowMe Beer's Law In Y=Mx+B Form A = ( l)c with the general form y =. The basic idea here is to use a graph plotting absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for. Beer's Law In Y=Mx+B Form.
From www.cuemath.com
y = mx + b What is Meaning of y = mx + b, How to Find Slope and Y Beer's Law In Y=Mx+B Form Once you have that you can compare the absorbance value of an. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A = ( l)c with the general form y =. The equation for beer’s law is a straight line with the general form of y = mx +b.. Beer's Law In Y=Mx+B Form.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law In Y=Mx+B Form A = ( l)c with the general form y =. You can also use this calculator to determine the. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The equation for beer’s law is a straight line with the general form of y = mx +b. The. Beer's Law In Y=Mx+B Form.
From www.coursehero.com
[Solved] When you turn beers law into y=mx+b, what components of beers Beer's Law In Y=Mx+B Form Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. You can also use this calculator to determine the. A = ( l)c with the general form y =. Once you have that you can compare the absorbance value of an. The basic idea here is to use a graph. Beer's Law In Y=Mx+B Form.
From www.slideserve.com
PPT CHM 5175 Part 2.3 PowerPoint Presentation, free download ID Beer's Law In Y=Mx+B Form Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The equation for beer’s law is a straight line with the general form of y = mx +b. The basic idea here is to use a graph plotting absorbance vs. Since the concentration, path length and molar absorptivity. Beer's Law In Y=Mx+B Form.
From www.showme.com
ShowMe solving for y in y = mx b Beer's Law In Y=Mx+B Form Once you have that you can compare the absorbance value of an. The equation for beer’s law is a straight line with the general form of y = mx +b. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. You can also use this calculator to determine the. The. Beer's Law In Y=Mx+B Form.
From www.cuemath.com
y = mx + b What is Meaning of y = mx + b, How to Find Slope and Y Beer's Law In Y=Mx+B Form Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. You can also use this calculator to determine the. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The basic idea here is to use a graph. Beer's Law In Y=Mx+B Form.
From worksheetideasbylinda.netlify.app
Y Mx B Worksheet Beer's Law In Y=Mx+B Form The equation for beer’s law is a straight line with the general form of y = mx +b. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions.. Beer's Law In Y=Mx+B Form.
From slideplayer.com
Absorbance spectroscopy ppt download Beer's Law In Y=Mx+B Form You can also use this calculator to determine the. Once you have that you can compare the absorbance value of an. A = ( l)c with the general form y =. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The equation for beer’s law is a straight line. Beer's Law In Y=Mx+B Form.
From www.youtube.com
Algebra basics graphing equation of a line in yintercept form y=mx+b Beer's Law In Y=Mx+B Form You can also use this calculator to determine the. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. The equation for beer’s law is a straight line. Beer's Law In Y=Mx+B Form.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law In Y=Mx+B Form The basic idea here is to use a graph plotting absorbance vs. The equation for beer’s law is a straight line with the general form of y = mx +b. Once you have that you can compare the absorbance value of an. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for. Beer's Law In Y=Mx+B Form.
From www.tessshebaylo.com
How To Solve For X In Y Mx B Equation Tessshebaylo Beer's Law In Y=Mx+B Form Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The equation for beer’s law is a straight line with the general form of y = mx +b. Once you have that you can compare the absorbance value of an. You can also use this calculator to determine. Beer's Law In Y=Mx+B Form.
From printablelibcoulter.z21.web.core.windows.net
What Represents Slope In Y Mx+b Beer's Law In Y=Mx+B Form The basic idea here is to use a graph plotting absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A = ( l)c with the general form y =. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for. Beer's Law In Y=Mx+B Form.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law In Y=Mx+B Form Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for two nearest standard solutions. The basic idea here is to use a graph plotting absorbance vs. A = ( l)c with the general form y =. You can also use this calculator to determine the. Once you have that you can compare. Beer's Law In Y=Mx+B Form.
From www.yumpu.com
How to Use Excel to Graph Your Maltose Standard Curve (y = mx + b) Beer's Law In Y=Mx+B Form Once you have that you can compare the absorbance value of an. The equation for beer’s law is a straight line with the general form of y = mx +b. A = ( l)c with the general form y =. The basic idea here is to use a graph plotting absorbance vs. Calculate the gradient (m) for the equation of. Beer's Law In Y=Mx+B Form.
From www.youtube.com
y=mx+b Explained y=mx+c YouTube Beer's Law In Y=Mx+B Form A = ( l)c with the general form y =. You can also use this calculator to determine the. The equation for beer’s law is a straight line with the general form of y = mx +b. Once you have that you can compare the absorbance value of an. Calculate the gradient (m) for the equation of the line of. Beer's Law In Y=Mx+B Form.
From printablelibcoulter.z21.web.core.windows.net
What Represents Slope In Y Mx+b Beer's Law In Y=Mx+B Form The basic idea here is to use a graph plotting absorbance vs. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following. A = ( l)c with the general form y =. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for. Beer's Law In Y=Mx+B Form.
From lessonlibconjoining.z22.web.core.windows.net
Y Mx B Worksheets Beer's Law In Y=Mx+B Form Once you have that you can compare the absorbance value of an. The basic idea here is to use a graph plotting absorbance vs. The equation for beer’s law is a straight line with the general form of y = mx +b. Calculate the gradient (m) for the equation of the line of the calibration curve, using the data for. Beer's Law In Y=Mx+B Form.