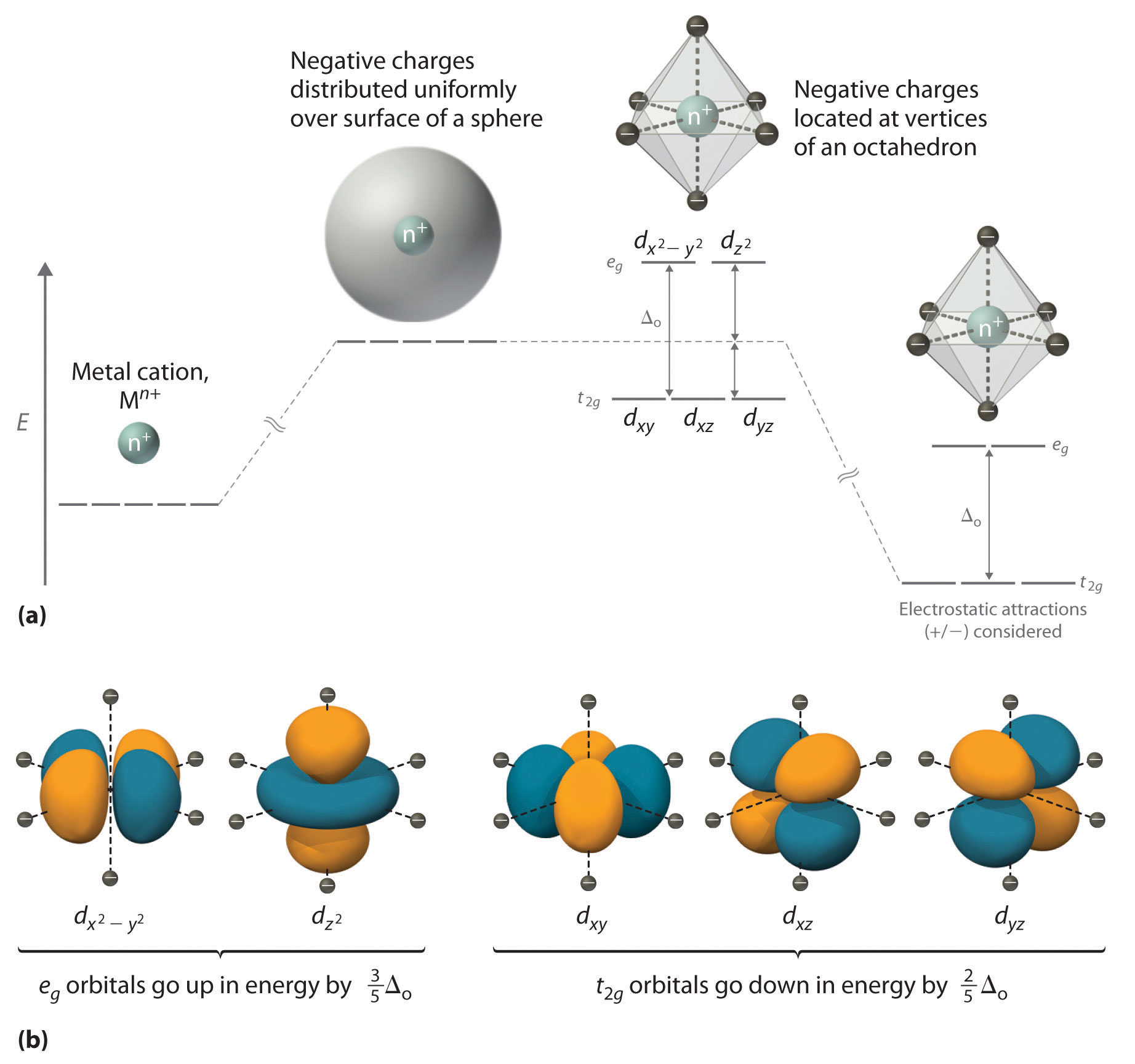
Draw Crystal Field Splitting For Octahedral Complex . If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. Crystal field theory and its applications to metal complexes 1. Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Draw and label the crystal field splitting diagram ( indicating filling of. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. See examples of square planar, tetrahedral, and octahedral. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude.
from chem.libretexts.org
See examples of square planar, tetrahedral, and octahedral. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. Crystal field theory and its applications to metal complexes 1. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Draw and label the crystal field splitting diagram ( indicating filling of. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds.
Introduction to Crystal Field Theory Chemistry LibreTexts
Draw Crystal Field Splitting For Octahedral Complex Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. Draw and label the crystal field splitting diagram ( indicating filling of. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. See examples of square planar, tetrahedral, and octahedral. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. Crystal field theory and its applications to metal complexes 1. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs.
From www.youtube.com
Crystal Field Theory PartII. Splitting in Tetrahedral, Square Planar Draw Crystal Field Splitting For Octahedral Complex Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. Crystal field theory and. Draw Crystal Field Splitting For Octahedral Complex.
From www.zigya.com
What do you understand by crystal field splitting? Draw figure to show Draw Crystal Field Splitting For Octahedral Complex Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. Crystal field theory and its applications to metal complexes 1. Learn about the crystal field splitting of d orbitals in. Draw Crystal Field Splitting For Octahedral Complex.
From www.researchgate.net
a) Splitting of the dorbital for a Cr 3+ cation in an octahedral Draw Crystal Field Splitting For Octahedral Complex If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Learn the basics of crystal field theory, the. Draw Crystal Field Splitting For Octahedral Complex.
From www.researchgate.net
Schematic representation of crystal field splitting for octahedral Draw Crystal Field Splitting For Octahedral Complex Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. See examples of square planar, tetrahedral, and octahedral. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g,. Draw Crystal Field Splitting For Octahedral Complex.
From scienceinfo.com
Crystal Field Theory Draw Crystal Field Splitting For Octahedral Complex Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. Draw and label the crystal field splitting diagram ( indicating filling of. Crystal field theory and its applications to metal complexes 1. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron. Draw Crystal Field Splitting For Octahedral Complex.
From www.jove.com
Crystal Field Theory Tetrahedral and Square Planar Complexes JoVE Draw Crystal Field Splitting For Octahedral Complex If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. Draw and label the crystal field splitting diagram ( indicating filling of. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the. Draw Crystal Field Splitting For Octahedral Complex.
From www.numerade.com
SOLVED Which of these crystal field splitting diagrams represent a low Draw Crystal Field Splitting For Octahedral Complex The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. Learn about the crystal field splitting of d orbitals in an octahedral. Draw Crystal Field Splitting For Octahedral Complex.
From www.toppr.com
The tetrahedral crystal field splitting is only.. of the octahedral Draw Crystal Field Splitting For Octahedral Complex The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. The difference in energy between the two sets of d orbitals is. Draw Crystal Field Splitting For Octahedral Complex.
From www.researchgate.net
Crystal field splitting of 3d orbitals in the tetrahedral, octahedral Draw Crystal Field Splitting For Octahedral Complex Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. The difference between the energy levels. Draw Crystal Field Splitting For Octahedral Complex.
From byjus.com
Crystal field stabilization energy for high spin d4 octahedral complex is Draw Crystal Field Splitting For Octahedral Complex The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. See examples of square planar, tetrahedral, and octahedral. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Draw and label the crystal field. Draw Crystal Field Splitting For Octahedral Complex.
From resolutionsforyou.com
Understanding Crystal Field Splitting Diagram for Octahedral Complexes Draw Crystal Field Splitting For Octahedral Complex See examples of square planar, tetrahedral, and octahedral. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. The difference in energy between the two sets. Draw Crystal Field Splitting For Octahedral Complex.
From www.meritnation.com
Why spliting of d orbital in tetrahedral crystal field different from Draw Crystal Field Splitting For Octahedral Complex Crystal field theory and its applications to metal complexes 1. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. Draw and. Draw Crystal Field Splitting For Octahedral Complex.
From www.teachoo.com
[Chemistry Class 12 SQP] Draw the crystal field splitting diagram for Draw Crystal Field Splitting For Octahedral Complex Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. Crystal field theory and its applications to metal complexes 1. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in. Draw Crystal Field Splitting For Octahedral Complex.
From chem.libretexts.org
Crystal Field Theory Chemistry LibreTexts Draw Crystal Field Splitting For Octahedral Complex See examples of square planar, tetrahedral, and octahedral. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in.. Draw Crystal Field Splitting For Octahedral Complex.
From app.jove.com
Crystal Field Theory Octahedral Complexes Concept Chemistry JoVe Draw Crystal Field Splitting For Octahedral Complex The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. Draw and label the crystal field splitting diagram ( indicating filling of. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs.. Draw Crystal Field Splitting For Octahedral Complex.
From www.researchgate.net
dOrbitals and Ligand Interactions using Octahedral field [19 Draw Crystal Field Splitting For Octahedral Complex Draw and label the crystal field splitting diagram ( indicating filling of. Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds.. Draw Crystal Field Splitting For Octahedral Complex.
From www.youtube.com
Trick for Crystal field theory (CFT) of Octahedral & Tetrahedral Draw Crystal Field Splitting For Octahedral Complex The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Crystal field theory and its applications to metal complexes 1. See examples of square planar, tetrahedral, and octahedral. Draw and label the crystal field splitting diagram ( indicating filling of. Learn about the ligand field. Draw Crystal Field Splitting For Octahedral Complex.
From www.toppr.com
Draw figure to show the splitting of d orbitals in an octahedral Draw Crystal Field Splitting For Octahedral Complex If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. The difference between the. Draw Crystal Field Splitting For Octahedral Complex.
From www.researchgate.net
Illustration of crystal field splitting in TMOs. A demonstrates Draw Crystal Field Splitting For Octahedral Complex The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. The difference in energy between the two sets of d orbitals is called the crystal field. Draw Crystal Field Splitting For Octahedral Complex.
From www.researchgate.net
a) Splitting of the dorbital for a Cr 3+ cation in an octahedral Draw Crystal Field Splitting For Octahedral Complex Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. Crystal field theory and its applications to metal complexes 1. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. Draw and label the crystal field. Draw Crystal Field Splitting For Octahedral Complex.
From www.researchgate.net
Ligand field splitting for Mo imp dorbitals produced by the Draw Crystal Field Splitting For Octahedral Complex Draw and label the crystal field splitting diagram ( indicating filling of. See examples of square planar, tetrahedral, and octahedral. Crystal field theory and its applications to metal complexes 1. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. The difference in energy between the two. Draw Crystal Field Splitting For Octahedral Complex.
From chem.libretexts.org
Introduction to Crystal Field Theory Chemistry LibreTexts Draw Crystal Field Splitting For Octahedral Complex Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. Crystal field theory and its applications to metal complexes 1. See examples of square planar, tetrahedral, and octahedral. Draw and label the crystal field splitting diagram ( indicating filling of. Learn about the crystal field splitting of d orbitals in an. Draw Crystal Field Splitting For Octahedral Complex.
From www.toppr.com
What is crystal field splitting energy? Draw a figure to show spitting Draw Crystal Field Splitting For Octahedral Complex Crystal field theory and its applications to metal complexes 1. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. The difference in energy between the two sets of d. Draw Crystal Field Splitting For Octahedral Complex.
From www.pdfprof.com
crystal field theory pdf Draw Crystal Field Splitting For Octahedral Complex The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. Draw and label the crystal field splitting diagram ( indicating filling of. Learn the basics of crystal field theory, the. Draw Crystal Field Splitting For Octahedral Complex.
From www.researchgate.net
Structural units, crystal field splitting in one unit complex and Draw Crystal Field Splitting For Octahedral Complex The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Draw and label the crystal field splitting diagram ( indicating filling of. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. Learn the basics of. Draw Crystal Field Splitting For Octahedral Complex.
From www.slideserve.com
PPT Crystal Field Theory (CFT) PowerPoint Presentation, free download Draw Crystal Field Splitting For Octahedral Complex Crystal field theory and its applications to metal complexes 1. See examples of square planar, tetrahedral, and octahedral. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Draw and label the crystal field splitting diagram ( indicating filling of. The difference between the energy. Draw Crystal Field Splitting For Octahedral Complex.
From www.numerade.com
SOLVED Draw out the crystal field splitting diagram for an octahedral Draw Crystal Field Splitting For Octahedral Complex Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. Draw and label the crystal field splitting diagram ( indicating filling of. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin. Draw Crystal Field Splitting For Octahedral Complex.
From app.jove.com
Crystal Field Theory Octahedral Complexes Chemistry JoVe Draw Crystal Field Splitting For Octahedral Complex If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs. Draw and label the crystal field splitting diagram ( indicating filling of. Crystal field theory and its applications to metal complexes 1. Learn about the ligand field theory and the crystal field theory. Draw Crystal Field Splitting For Octahedral Complex.
From www.mikrora.com
Crystal Field Splitting Diagram Draw Crystal Field Splitting For Octahedral Complex See examples of square planar, tetrahedral, and octahedral. Draw and label the crystal field splitting diagram ( indicating filling of. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. The difference between the energy levels in an octahedral complex is called the crystal field splitting energy (δ o), whose magnitude.. Draw Crystal Field Splitting For Octahedral Complex.
From 2012books.lardbucket.org
Crystal Field Theory Draw Crystal Field Splitting For Octahedral Complex The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Crystal field theory and its applications to metal complexes 1. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin. Draw Crystal Field Splitting For Octahedral Complex.
From galvinconanstuart.blogspot.com
Draw The Octahedral Crystal Field Splitting Diagram For Each Metal Ion Draw Crystal Field Splitting For Octahedral Complex The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Learn about the ligand field theory and the crystal field theory that explain the spin states of coordination compounds. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the. Draw Crystal Field Splitting For Octahedral Complex.
From www.chegg.com
Solved The general crystal field splitting of an octahedral Draw Crystal Field Splitting For Octahedral Complex See examples of square planar, tetrahedral, and octahedral. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. If the energy required to pair two electrons is greater than δ, the energy cost of placing an electron in an e g, high spin splitting occurs.. Draw Crystal Field Splitting For Octahedral Complex.
From chemistry.stackexchange.com
transition metals Octahedral Crystal Field Splitting Orbital Energy Draw Crystal Field Splitting For Octahedral Complex Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and. Crystal field theory and its applications to metal complexes 1. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. If the. Draw Crystal Field Splitting For Octahedral Complex.
From app.jove.com
Crystal Field Theory Tetrahedral and Square Planar Complexes Draw Crystal Field Splitting For Octahedral Complex Draw and label the crystal field splitting diagram ( indicating filling of. Crystal field theory and its applications to metal complexes 1. Learn about the crystal field splitting of d orbitals in an octahedral field, the factors affecting the magnitude of δ, the spectrochemical series, and the distribution of electrons in. Learn the basics of crystal field theory, the interaction. Draw Crystal Field Splitting For Octahedral Complex.
From ar.inspiredpencil.com
D Orbital Splitting Draw Crystal Field Splitting For Octahedral Complex Draw and label the crystal field splitting diagram ( indicating filling of. The difference in energy between the two sets of d orbitals is called the crystal field splitting energy (\(\delta_o\), where the subscript o stands for. Learn the basics of crystal field theory, the interaction of metal ions and ligands, and the splitting of d orbitals in octahedral and.. Draw Crystal Field Splitting For Octahedral Complex.