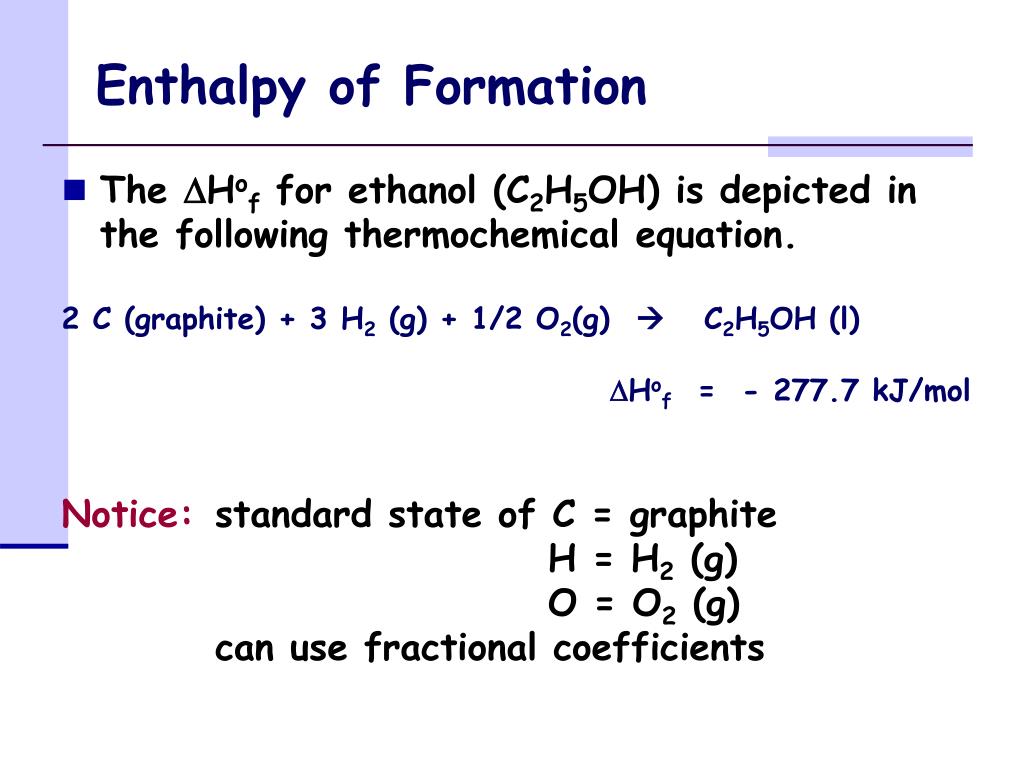
Nitrogen Oxide Enthalpy Of Formation . O = a degree signifies that it's a standard enthalpy change. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. 21 rows cse division home. The symbol of the standard enthalpy of formation is δh f. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. Δ = a change in enthalpy;
from mungfali.com
For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. O = a degree signifies that it's a standard enthalpy change. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 21 rows cse division home. Δ = a change in enthalpy; The symbol of the standard enthalpy of formation is δh f.
Standard Enthalpy Of Formation Equation
Nitrogen Oxide Enthalpy Of Formation Δ = a change in enthalpy; Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero by definition. Δ = a change in enthalpy; Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. O = a degree signifies that it's a standard enthalpy change. 21 rows cse division home. The symbol of the standard enthalpy of formation is δh f. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas Enthalpy Of Formation Nitrogen Oxide Enthalpy Of Formation The symbol of the standard enthalpy of formation is δh f. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. 21 rows cse division home. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation. Nitrogen Oxide Enthalpy Of Formation.
From byjus.com
70. The standard enthalpy of formation of FeO and Fe2O3 is 65 kcal mol Nitrogen Oxide Enthalpy Of Formation 21 rows cse division home. Δ = a change in enthalpy; Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Nitrogen Oxide Enthalpy Of Formation.
From mungfali.com
Standard Enthalpy Of Formation Equation Nitrogen Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. The standard enthalpy of formation of any element in its standard state is zero by definition. The symbol of. Nitrogen Oxide Enthalpy Of Formation.
From www.slideserve.com
PPT NITROGENOXIDES PowerPoint Presentation, free download ID4735299 Nitrogen Oxide Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. Δ = a change in enthalpy; The symbol of the standard enthalpy of formation is δh f. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Upon condensing to a. Nitrogen Oxide Enthalpy Of Formation.
From mungfali.com
Enthalpies Of Formation Chart Nitrogen Oxide Enthalpy Of Formation Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. 21 rows cse division home. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Selected. Nitrogen Oxide Enthalpy Of Formation.
From www.researchgate.net
Standard enthalpy of formation from binary oxides vs tolerance factor Nitrogen Oxide Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The symbol of. Nitrogen Oxide Enthalpy Of Formation.
From www.slideshare.net
Oxide of nitrogen Nitrogen Oxide Enthalpy Of Formation 21 rows cse division home. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Δ = a change in enthalpy; Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Nitrogen Oxide Enthalpy Of Formation.
From www.chegg.com
TABLE A286 Enthalpy of formation, Gibbs function of Nitrogen Oxide Enthalpy Of Formation O = a degree signifies that it's a standard enthalpy change. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the. Nitrogen Oxide Enthalpy Of Formation.
From www.engineeringtoolbox.com
Nitrogen Enthalpy, Internal Energy and Entropy vs. Temperature Nitrogen Oxide Enthalpy Of Formation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero by definition. The symbol of the standard enthalpy of formation is δh f. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. O =. Nitrogen Oxide Enthalpy Of Formation.
From classhirsch.z21.web.core.windows.net
Standard Enthalpy Of Formation Chart Nitrogen Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Δ = a change in enthalpy; 21 rows cse division home. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Selected atct [1, 2] enthalpy. Nitrogen Oxide Enthalpy Of Formation.
From www.researchgate.net
The enthalpy of formation −ΔHf of the oxide per mole of O for some Nitrogen Oxide Enthalpy Of Formation The standard enthalpy of formation of any element in its standard state is zero by definition. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. The symbol of the standard enthalpy of formation is δh f. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is. Nitrogen Oxide Enthalpy Of Formation.
From www.researchgate.net
Enthalpy of formation, reaction, and of metal oxides and Nitrogen Oxide Enthalpy Of Formation Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. O = a degree signifies that it's a standard enthalpy change. Δ = a change in enthalpy; The symbol of the standard enthalpy of. Nitrogen Oxide Enthalpy Of Formation.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Nitrogen Oxide Enthalpy Of Formation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. 21 rows cse division home. 193 rows in chemistry and thermodynamics,. Nitrogen Oxide Enthalpy Of Formation.
From www.fatherskit.co
combustion of glucose enthalpy calculate the heat of combustion Nitrogen Oxide Enthalpy Of Formation The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Selected atct [1, 2] enthalpy of formation based. Nitrogen Oxide Enthalpy Of Formation.
From www.youtube.com
The enthalpy changes of formation of the gaseous oxides of nitrogen `(N Nitrogen Oxide Enthalpy Of Formation The symbol of the standard enthalpy of formation is δh f. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 21 rows cse division home. Δ = a change in. Nitrogen Oxide Enthalpy Of Formation.
From courses.lumenlearning.com
9.3 Enthalpy General College Chemistry I Nitrogen Oxide Enthalpy Of Formation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. O = a degree signifies that it's a. Nitrogen Oxide Enthalpy Of Formation.
From pdfprof.com
enthalpies standard de formation et entropie standard Nitrogen Oxide Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. The symbol of the standard enthalpy of formation is δh f. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen. Nitrogen Oxide Enthalpy Of Formation.
From www.numerade.com
SOLVED 13. Calculate the enthalpy for the formation of calcium Nitrogen Oxide Enthalpy Of Formation 21 rows cse division home. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Δ = a change in enthalpy; Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network. Nitrogen Oxide Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Nitrogen Oxide Enthalpy Of Formation Δ = a change in enthalpy; 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. 21 rows cse division home. O = a degree signifies that it's a standard enthalpy change. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The symbol. Nitrogen Oxide Enthalpy Of Formation.
From www.numerade.com
SOLVED(a) The nitrogen atoms in an N2 molecule are held together by a Nitrogen Oxide Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. The symbol of the standard enthalpy of formation. Nitrogen Oxide Enthalpy Of Formation.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Nitrogen Oxide Enthalpy Of Formation Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. The. Nitrogen Oxide Enthalpy Of Formation.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Nitrogen Oxide Enthalpy Of Formation 21 rows cse division home. The symbol of the standard enthalpy of formation is δh f. O = a degree signifies that it's a standard enthalpy change. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Δ = a change in enthalpy; The standard enthalpy of formation of. Nitrogen Oxide Enthalpy Of Formation.
From www.slideshare.net
Standard enthalpy of formation Nitrogen Oxide Enthalpy Of Formation The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. O = a degree signifies that it's a standard enthalpy change. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Nitrogen Oxide Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Nitrogen Oxide Enthalpy Of Formation Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of. Nitrogen Oxide Enthalpy Of Formation.
From www.chegg.com
Solved Gaseous Nitrogen Dioxide NO2, Reacts With Oxygen T... Nitrogen Oxide Enthalpy Of Formation Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero by definition. 21 rows cse division home. Selected atct [1, 2] enthalpy of formation based. Nitrogen Oxide Enthalpy Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Nitrogen Oxide Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Δ = a change in enthalpy; 21 rows cse division home. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen. Nitrogen Oxide Enthalpy Of Formation.
From mavink.com
Enthalpy Of Formation Symbol Nitrogen Oxide Enthalpy Of Formation 21 rows cse division home. Δ = a change in enthalpy; Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Nitrogen Oxide Enthalpy Of Formation.
From www.chegg.com
Solved The chemistry of nitrogen oxides is very versatile. Nitrogen Oxide Enthalpy Of Formation 21 rows cse division home. The symbol of the standard enthalpy of formation is δh f. O = a degree signifies that it's a standard enthalpy change. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Δ = a change in enthalpy; Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical. Nitrogen Oxide Enthalpy Of Formation.
From lectio.info
Heat Of Formation Of Mgo Nitrogen Oxide Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The symbol of the standard enthalpy of formation is δh f. The standard enthalpy of formation of any element in its. Nitrogen Oxide Enthalpy Of Formation.
From pt.slideshare.net
Tang 03 enthalpy of formation and combustion Nitrogen Oxide Enthalpy Of Formation The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. O = a degree signifies that it's a standard enthalpy change. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources,. Nitrogen Oxide Enthalpy Of Formation.
From www.chegg.com
Solved The chemistry of nitrogen oxides is very versatile. Nitrogen Oxide Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Δ = a change in enthalpy; Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Nitrogen Oxide Enthalpy Of Formation.
From classnotes.org.in
Oxides of Nitrogen Chemistry, Class 12, The pBlock Elements Nitrogen Oxide Enthalpy Of Formation Selected atct [1, 2] enthalpy of formation based on version 1.118 of the thermochemical network [3] this version. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The symbol of the standard enthalpy of formation is δh f. The standard enthalpy of formation of any element. Nitrogen Oxide Enthalpy Of Formation.
From www.chegg.com
Solved Nitric oxide (NO) can be formed from nitrogen, Nitrogen Oxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Upon condensing to a liquid, nitric oxide dimerizes to dinitrogen dioxide, but the association is weak and reversible. The symbol of the standard enthalpy. Nitrogen Oxide Enthalpy Of Formation.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Nitrogen Oxide Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 21 rows cse division home. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the. Nitrogen Oxide Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Nitrogen Oxide Enthalpy Of Formation For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Δ = a change in enthalpy; 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. Upon condensing. Nitrogen Oxide Enthalpy Of Formation.