Specific Heat Calorimetry Lab Answers . specific heat capacity can be described as a substance's resistance to temperature changes. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. heat is a form of energy that is transferred between objects with different temperatures. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? determine specific heats (or heats of chemical reactions) is called a calorimeter. Which substance has a greater. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. (specific heat of aluminum = 0 j/g°c). study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses.
from www.studocu.com
determine specific heats (or heats of chemical reactions) is called a calorimeter. Which substance has a greater. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. heat is a form of energy that is transferred between objects with different temperatures. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. (specific heat of aluminum = 0 j/g°c). calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. specific heat capacity can be described as a substance's resistance to temperature changes. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,.
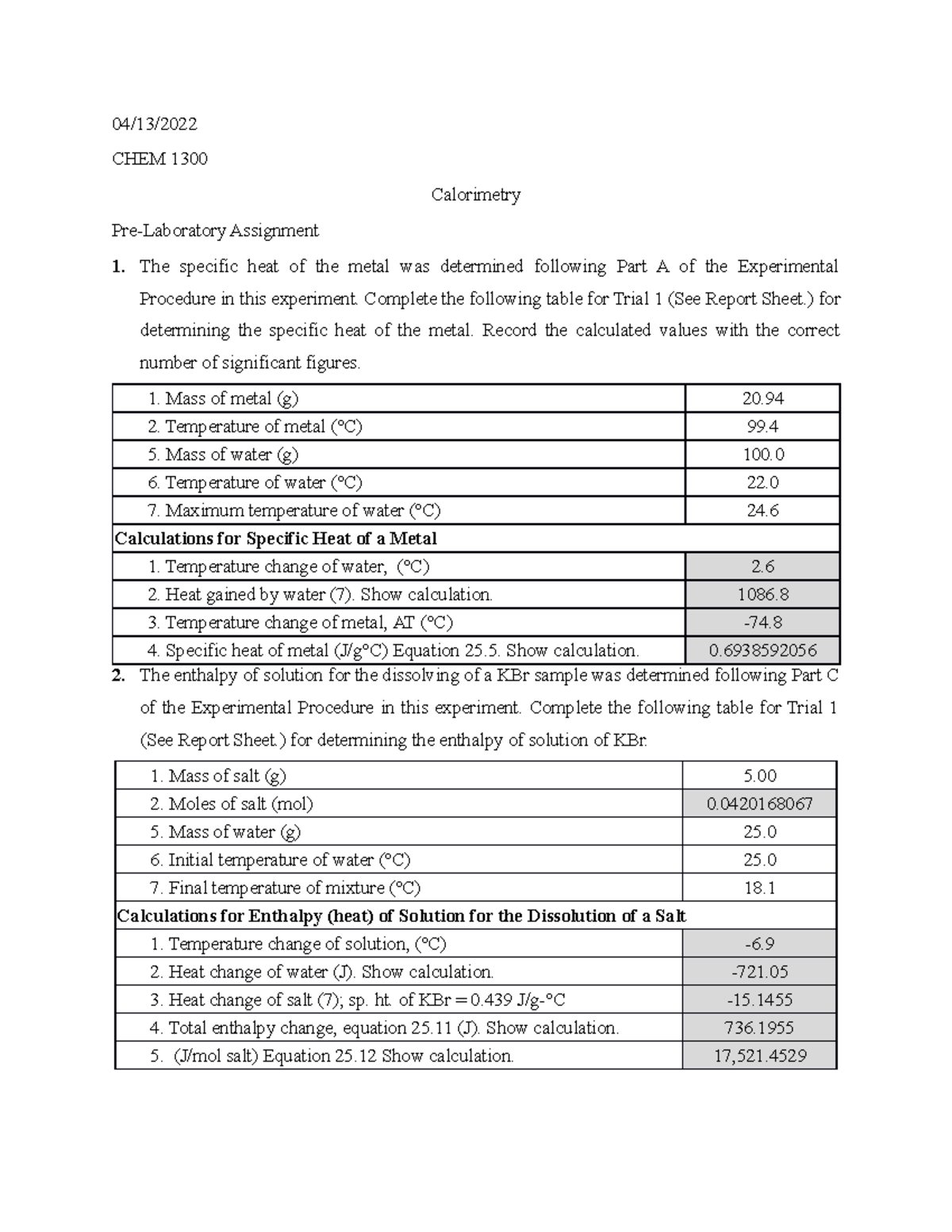
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Assignment 1. The specific
Specific Heat Calorimetry Lab Answers A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. heat is a form of energy that is transferred between objects with different temperatures. (specific heat of aluminum = 0 j/g°c). determine specific heats (or heats of chemical reactions) is called a calorimeter. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. specific heat capacity can be described as a substance's resistance to temperature changes. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? Which substance has a greater. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a.
From kimora-kbowen.blogspot.com
Temperature and Specific Heat Lab 4 Answers Specific Heat Calorimetry Lab Answers in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. heat is a form of energy that is transferred between objects with different temperatures. Which substance has a greater. calculate the amount. Specific Heat Calorimetry Lab Answers.
From afashionlista.blogspot.com
Answer Key Calorimetry Lab Gizmo Answers Activity C / Explore Learning Circuits Gizmo Activity C Specific Heat Calorimetry Lab Answers study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. (specific heat of aluminum = 0 j/g°c). Which substance has a greater. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? determine specific heats (or heats. Specific Heat Calorimetry Lab Answers.
From www.studocu.com
Calorimetry Part 1 Specific Heat Capacity Chem 200 Lab Report Calorimetry Part 1 Specific Specific Heat Calorimetry Lab Answers A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? determine specific heats. Specific Heat Calorimetry Lab Answers.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Docsity Specific Heat Calorimetry Lab Answers Which substance has a greater. determine specific heats (or heats of chemical reactions) is called a calorimeter. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a.. Specific Heat Calorimetry Lab Answers.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Specific Heat Calorimetry Lab Answers determine specific heats (or heats of chemical reactions) is called a calorimeter. (specific heat of aluminum = 0 j/g°c). calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c?. Specific Heat Calorimetry Lab Answers.
From learninglibraryralf.z13.web.core.windows.net
Specific Heat And Calorimetry Worksheet Specific Heat Calorimetry Lab Answers determine specific heats (or heats of chemical reactions) is called a calorimeter. specific heat capacity can be described as a substance's resistance to temperature changes. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. (specific heat of aluminum = 0 j/g°c). calculate the amount of heat transferred from the engine to the. Specific Heat Calorimetry Lab Answers.
From studylib.net
Calorimetry Lab Specific Heat Capacity Specific Heat Calorimetry Lab Answers heat is a form of energy that is transferred between objects with different temperatures. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. (specific heat of. Specific Heat Calorimetry Lab Answers.
From www.chegg.com
Laboratory 9 CALORIMETRY SPECIFIC HEAT OF A METAL Specific Heat Calorimetry Lab Answers specific heat capacity can be described as a substance's resistance to temperature changes. (specific heat of aluminum = 0 j/g°c). A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. Which substance has a greater. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c?. Specific Heat Calorimetry Lab Answers.
From www.chegg.com
Solved Calorimetry Lab Report o PART A. Specific Heat Specific Heat Calorimetry Lab Answers Which substance has a greater. determine specific heats (or heats of chemical reactions) is called a calorimeter. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and.. Specific Heat Calorimetry Lab Answers.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie calorimeter joule specific heat Specific Heat Calorimetry Lab Answers How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. specific heat capacity can be described as a substance's resistance to temperature changes. determine specific heats (or heats of chemical reactions) is called a. Specific Heat Calorimetry Lab Answers.
From www.numerade.com
SOLVED In the laboratory a "coffee cup calorimeter, Or constant pressure calorimeter; is Specific Heat Calorimetry Lab Answers calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. specific heat capacity can be described as a substance's resistance to temperature changes. Which substance has a greater. determine specific heats (or heats of chemical reactions) is called a calorimeter. How much heat energy is needed to raise. Specific Heat Calorimetry Lab Answers.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Assignment 1. The specific Specific Heat Calorimetry Lab Answers specific heat capacity can be described as a substance's resistance to temperature changes. Which substance has a greater. (specific heat of aluminum = 0 j/g°c). study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. in this set of practice questions, we will go over the. Specific Heat Calorimetry Lab Answers.
From brainly.com
please i need it Lab Calorimetry and Specific Heat Specific Heat Calorimetry Lab Answers How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? Which substance has a greater. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. . Specific Heat Calorimetry Lab Answers.
From learninglibrarylinton.z21.web.core.windows.net
Calorimetry Questions And Answers Specific Heat Calorimetry Lab Answers (specific heat of aluminum = 0 j/g°c). heat is a form of energy that is transferred between objects with different temperatures. determine specific heats (or heats of chemical reactions) is called a calorimeter. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. A perfect calorimeter absorbs no. Specific Heat Calorimetry Lab Answers.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Specific Heat Calorimetry Lab Answers (specific heat of aluminum = 0 j/g°c). study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. specific heat capacity can be described as a substance's resistance to temperature changes. determine specific heats (or heats of chemical reactions) is called a calorimeter. Which substance has a. Specific Heat Calorimetry Lab Answers.
From www.chegg.com
Solved REPORT FOR EXPERIMENT5 Calorimetry and Specific Heat Specific Heat Calorimetry Lab Answers in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? specific heat capacity can be described as a substance's resistance to temperature changes. determine specific heats. Specific Heat Calorimetry Lab Answers.
From stockautumn12.blogspot.com
Calorimetry Lab Worksheet Answer Key Calorimetry Gizmo Lab Name Date Student Exploration Specific Heat Calorimetry Lab Answers How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. Which substance has a greater. heat is a form of energy that is transferred between objects with. Specific Heat Calorimetry Lab Answers.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter, joule, specific heat Specific Heat Calorimetry Lab Answers in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. heat. Specific Heat Calorimetry Lab Answers.
From studyschoolburman.z21.web.core.windows.net
Heat And Calorimetry Worksheet Answer Key Specific Heat Calorimetry Lab Answers (specific heat of aluminum = 0 j/g°c). calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. specific heat capacity can be described as a substance's resistance to temperature changes. in this set of practice questions, we will go over the main types of questions on calorimetry including. Specific Heat Calorimetry Lab Answers.
From studylib.net
Lab 11b Specific Heat & Heat Capacity Specific Heat Calorimetry Lab Answers Which substance has a greater. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. (specific heat of aluminum = 0 j/g°c). calculate the amount of heat. Specific Heat Calorimetry Lab Answers.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Specific Heat Calorimetry Lab Answers determine specific heats (or heats of chemical reactions) is called a calorimeter. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. specific heat capacity can be described as a substance's resistance to temperature changes. calculate the amount of heat transferred from the engine to. Specific Heat Calorimetry Lab Answers.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Specific Heat Calorimetry Lab Answers How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? (specific heat of aluminum = 0 j/g°c). in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calculate the amount of heat transferred from the engine to. Specific Heat Calorimetry Lab Answers.
From studylib.net
Calorimetry Worksheet Specific Heat Calorimetry Lab Answers heat is a form of energy that is transferred between objects with different temperatures. Which substance has a greater. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color,. Specific Heat Calorimetry Lab Answers.
From www.chegg.com
Solved Report on Laboratory Experiment "Specific Heat of a Specific Heat Calorimetry Lab Answers in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. specific heat capacity can be described as a substance's resistance to temperature changes. How much. Specific Heat Calorimetry Lab Answers.
From www.chegg.com
Solved LAB REPORT CALORIMETRY Table 11.1 Specific heat. Specific Heat Calorimetry Lab Answers specific heat capacity can be described as a substance's resistance to temperature changes. determine specific heats (or heats of chemical reactions) is called a calorimeter. heat is a form of energy that is transferred between objects with different temperatures. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. calculate the amount. Specific Heat Calorimetry Lab Answers.
From www.scribd.com
Hand Out 1.5 Specific Heat Calorimetry Lab PDF Heat Calorimetry Specific Heat Calorimetry Lab Answers calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. heat is a form of energy that is transferred between objects with different temperatures. How much heat energy is needed to raise the temperature of. Specific Heat Calorimetry Lab Answers.
From www.chegg.com
Solved Calorimetry Heat of Fusion and Specific Heat(we are Specific Heat Calorimetry Lab Answers (specific heat of aluminum = 0 j/g°c). specific heat capacity can be described as a substance's resistance to temperature changes. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in. Specific Heat Calorimetry Lab Answers.
From phonebay1.blogspot.com
Check Student Exploration Calorimetry Lab Answer Key Updated 2021 Phone Bay Specific Heat Calorimetry Lab Answers Which substance has a greater. heat is a form of energy that is transferred between objects with different temperatures. (specific heat of aluminum = 0 j/g°c). in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. calculate the amount of heat transferred from the engine to. Specific Heat Calorimetry Lab Answers.
From www.coursehero.com
[Solved] Lab 6 Specific Heats and Calorimetry General Chemistry I Prof.... Course Hero Specific Heat Calorimetry Lab Answers A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. in this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. calculate. Specific Heat Calorimetry Lab Answers.
From sfsgyfbhpf.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Calorimetrylabse Pdf Daniel Lara Name Date Student Specific Heat Calorimetry Lab Answers (specific heat of aluminum = 0 j/g°c). study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. determine specific heats (or heats of chemical reactions) is called a calorimeter. heat is a form of energy that is transferred between objects with different temperatures. A perfect calorimeter. Specific Heat Calorimetry Lab Answers.
From www.studypool.com
SOLUTION Phys1020 unit 5 worksheet 1 specific heat calorimetry Studypool Specific Heat Calorimetry Lab Answers determine specific heats (or heats of chemical reactions) is called a calorimeter. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. in this set of practice questions, we will go over the main. Specific Heat Calorimetry Lab Answers.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Specific Heat Calorimetry Lab Answers Which substance has a greater. determine specific heats (or heats of chemical reactions) is called a calorimeter. study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. calculate the amount of heat. Specific Heat Calorimetry Lab Answers.
From www.transtutors.com
(Solved) Data Table For The Specific Heat And Calorimeter Lab Specific Heat... (1 Answer Specific Heat Calorimetry Lab Answers heat is a form of energy that is transferred between objects with different temperatures. How much heat energy is needed to raise the temperature of a 55g sample of aluminum from 22°c to 94°c? calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. in this set of. Specific Heat Calorimetry Lab Answers.
From www.numerade.com
SOLVED In the laboratory a "coffee cup" calorimeter, Or constant pressure calorimeter; is Specific Heat Calorimetry Lab Answers study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. A perfect calorimeter absorbs no heat from the solution that it contains, nor loses. (specific heat of aluminum = 0 j/g°c). How much heat energy is needed to raise the temperature of a 55g sample of aluminum from. Specific Heat Calorimetry Lab Answers.
From www.studypool.com
SOLUTION Gizmo student exploration calorimetry lab answer key Studypool Specific Heat Calorimetry Lab Answers (specific heat of aluminum = 0 j/g°c). determine specific heats (or heats of chemical reactions) is called a calorimeter. calculate the amount of heat transferred from the engine to the surroundings by one gallon of water with a. specific heat capacity can be described as a substance's resistance to temperature changes. study with quizlet and memorize. Specific Heat Calorimetry Lab Answers.