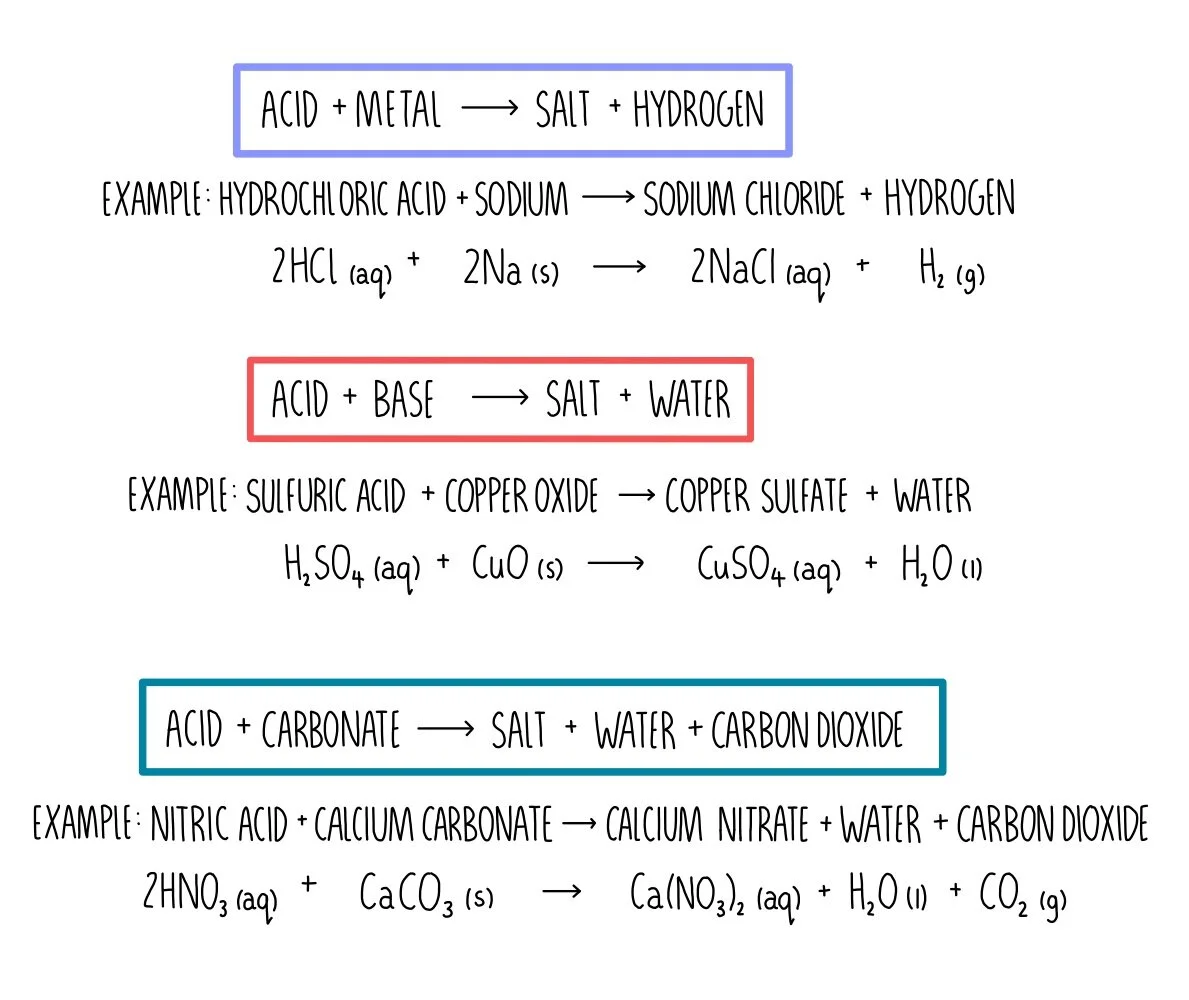
What Happens If You Add A Base To An Acid . An acid and a base are like chemical opposites. Moreover, many of the substances we encounter in our homes,. when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. adding a base decreases the concentration of h 3 o + ions in the solution. This keeps the ph relatively stable. If a base is added to an. acids are substances that ionize to form hydrogen ions in solution. How do acids and bases neutralize one another (or cancel each other out)? Bases ionize for form hydroxy ions in solution. The product of such a.
from www.thesciencehive.co.uk
The product of such a. How do acids and bases neutralize one another (or cancel each other out)? acids are substances that ionize to form hydrogen ions in solution. Moreover, many of the substances we encounter in our homes,. An acid and a base are like chemical opposites. when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. adding a base decreases the concentration of h 3 o + ions in the solution. If a base is added to an. Bases ionize for form hydroxy ions in solution. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or.
Acids, Bases and Salt Preparations (GCSE) — the science hive
What Happens If You Add A Base To An Acid the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. The product of such a. adding a base decreases the concentration of h 3 o + ions in the solution. Moreover, many of the substances we encounter in our homes,. when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. An acid and a base are like chemical opposites. How do acids and bases neutralize one another (or cancel each other out)? Bases ionize for form hydroxy ions in solution. acids are substances that ionize to form hydrogen ions in solution. If a base is added to an. This keeps the ph relatively stable. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or.
From brainly.in
what happens when we add acid and base on phenolphthalein?? Brainly.in What Happens If You Add A Base To An Acid Moreover, many of the substances we encounter in our homes,. if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. The product of such a. acids are substances that ionize to form hydrogen ions in solution. How do acids and bases neutralize one another (or cancel each other. What Happens If You Add A Base To An Acid.
From www.youtube.com
what happens to an acid or base in a water solution? class 10th What Happens If You Add A Base To An Acid Moreover, many of the substances we encounter in our homes,. An acid and a base are like chemical opposites. How do acids and bases neutralize one another (or cancel each other out)? adding a base decreases the concentration of h 3 o + ions in the solution. the added \ (hcl\) (a strong acid) or \ (naoh\) (a. What Happens If You Add A Base To An Acid.
From studytomikamariepc.z14.web.core.windows.net
How To Identify Acids And Bases In A Equation What Happens If You Add A Base To An Acid adding a base decreases the concentration of h 3 o + ions in the solution. How do acids and bases neutralize one another (or cancel each other out)? This keeps the ph relatively stable. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. Bases ionize. What Happens If You Add A Base To An Acid.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases What Happens If You Add A Base To An Acid How do acids and bases neutralize one another (or cancel each other out)? if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. Moreover, many of. What Happens If You Add A Base To An Acid.
From dxorbotyq.blob.core.windows.net
What Happens If You Add Water To A Base at Joseph Bass blog What Happens If You Add A Base To An Acid if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. Bases ionize for form hydroxy ions in solution. adding a base decreases the concentration of h 3 o + ions in the solution. This keeps the ph relatively stable. the added \ (hcl\) (a strong acid) or. What Happens If You Add A Base To An Acid.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW What Happens If You Add A Base To An Acid the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. Bases ionize for form hydroxy ions in solution. Moreover, many of the substances we encounter in our homes,. How do acids and bases neutralize one another (or cancel each other out)? The product of such a. . What Happens If You Add A Base To An Acid.
From www.kidsplayandcreate.com
What Happens When you Mix an Acid with a Base? Kids Science Kids Play What Happens If You Add A Base To An Acid If a base is added to an. How do acids and bases neutralize one another (or cancel each other out)? adding a base decreases the concentration of h 3 o + ions in the solution. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. . What Happens If You Add A Base To An Acid.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089385 What Happens If You Add A Base To An Acid The product of such a. Moreover, many of the substances we encounter in our homes,. How do acids and bases neutralize one another (or cancel each other out)? when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. acids are substances that ionize to form hydrogen ions in solution.. What Happens If You Add A Base To An Acid.
From thechemistrynotes.com
AcidBase Reaction (Neutralization Reaction) What Happens If You Add A Base To An Acid when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. How do acids and bases neutralize one another (or cancel each other out)? If a base is added to an. adding a base decreases the concentration of h 3 o + ions in the solution. Bases ionize for form. What Happens If You Add A Base To An Acid.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses What Happens If You Add A Base To An Acid An acid and a base are like chemical opposites. Moreover, many of the substances we encounter in our homes,. acids are substances that ionize to form hydrogen ions in solution. when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. Bases ionize for form hydroxy ions in solution. . What Happens If You Add A Base To An Acid.
From www.youtube.com
Mixing Strong Concentrated Acid and Base YouTube What Happens If You Add A Base To An Acid acids are substances that ionize to form hydrogen ions in solution. Bases ionize for form hydroxy ions in solution. How do acids and bases neutralize one another (or cancel each other out)? This keeps the ph relatively stable. adding a base decreases the concentration of h 3 o + ions in the solution. the added \ (hcl\). What Happens If You Add A Base To An Acid.
From www.youtube.com
Identifying Acids, Bases, & Conjugate Acids & Bases in an Acid/Base What Happens If You Add A Base To An Acid when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. The product of such a. if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. An acid and a base are like chemical opposites. This keeps the ph relatively. What Happens If You Add A Base To An Acid.
From morioh.com
Acids and Bases Basic Introduction Chemistry What Happens If You Add A Base To An Acid How do acids and bases neutralize one another (or cancel each other out)? Moreover, many of the substances we encounter in our homes,. An acid and a base are like chemical opposites. acids are substances that ionize to form hydrogen ions in solution. if you mix equal amounts of a strong acid and a strong base, the two. What Happens If You Add A Base To An Acid.
From www.youtube.com
What Happens to an Acid or a Base in Water Solution Class 10 What Happens If You Add A Base To An Acid If a base is added to an. Moreover, many of the substances we encounter in our homes,. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. This keeps the ph relatively stable. An acid and a base are like chemical opposites. if you mix equal. What Happens If You Add A Base To An Acid.
From www.dreamstime.com
Acid base reaction stock vector. Illustration of chemistry 200906334 What Happens If You Add A Base To An Acid adding a base decreases the concentration of h 3 o + ions in the solution. This keeps the ph relatively stable. Moreover, many of the substances we encounter in our homes,. The product of such a. An acid and a base are like chemical opposites. if you mix equal amounts of a strong acid and a strong base,. What Happens If You Add A Base To An Acid.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo What Happens If You Add A Base To An Acid This keeps the ph relatively stable. An acid and a base are like chemical opposites. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. Bases ionize for form hydroxy ions in solution. Moreover, many of the substances we encounter in our homes,. How do acids and. What Happens If You Add A Base To An Acid.
From www.youtube.com
Chemistry Acid or base in water solution Acids, bases and salts What Happens If You Add A Base To An Acid if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. acids are substances that ionize to form hydrogen ions in solution. If a base is added to an. How do acids and bases neutralize one another (or cancel each other out)? adding a base decreases the concentration. What Happens If You Add A Base To An Acid.
From evulpo.com
Acids and bases Chemistry Explanation & Exercises evulpo What Happens If You Add A Base To An Acid if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. Bases ionize for form hydroxy ions in solution. acids are substances that ionize to form hydrogen ions in solution. The product of such a. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base). What Happens If You Add A Base To An Acid.
From arielle-well-wolfe.blogspot.com
Chemistry Form 4 Chapter 7 Acid and Base What Happens If You Add A Base To An Acid Moreover, many of the substances we encounter in our homes,. If a base is added to an. How do acids and bases neutralize one another (or cancel each other out)? This keeps the ph relatively stable. The product of such a. An acid and a base are like chemical opposites. if you mix equal amounts of a strong acid. What Happens If You Add A Base To An Acid.
From www.youtube.com
What happens to and Acid or Base in Water Solution?Acid Bases and What Happens If You Add A Base To An Acid The product of such a. This keeps the ph relatively stable. If a base is added to an. How do acids and bases neutralize one another (or cancel each other out)? Bases ionize for form hydroxy ions in solution. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak. What Happens If You Add A Base To An Acid.
From www.youtube.com
Acids and Bases Reaction with each other Don't Memorise YouTube What Happens If You Add A Base To An Acid Moreover, many of the substances we encounter in our homes,. acids are substances that ionize to form hydrogen ions in solution. If a base is added to an. This keeps the ph relatively stable. if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. when you add. What Happens If You Add A Base To An Acid.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs What Happens If You Add A Base To An Acid acids are substances that ionize to form hydrogen ions in solution. Moreover, many of the substances we encounter in our homes,. Bases ionize for form hydroxy ions in solution. when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. The product of such a. If a base is added. What Happens If You Add A Base To An Acid.
From dxozctuig.blob.core.windows.net
Base Definition Chemistry Gcse at Era Dawson blog What Happens If You Add A Base To An Acid Bases ionize for form hydroxy ions in solution. If a base is added to an. Moreover, many of the substances we encounter in our homes,. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. This keeps the ph relatively stable. adding a base decreases the. What Happens If You Add A Base To An Acid.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID What Happens If You Add A Base To An Acid How do acids and bases neutralize one another (or cancel each other out)? if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. This keeps the ph relatively stable. acids are substances that ionize to form hydrogen ions in solution. the added \ (hcl\) (a strong acid). What Happens If You Add A Base To An Acid.
From www.masterorganicchemistry.com
Introduction to AcidBase Reactions Master Organic Chemistry What Happens If You Add A Base To An Acid The product of such a. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. Moreover, many of the substances we encounter in our homes,. acids are substances that ionize to form hydrogen ions in solution. If a base is added to an. if you. What Happens If You Add A Base To An Acid.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID What Happens If You Add A Base To An Acid This keeps the ph relatively stable. If a base is added to an. if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. How do acids and bases neutralize one another (or cancel each other out)? The product of such a. adding a base decreases the concentration of. What Happens If You Add A Base To An Acid.
From dxotrudlt.blob.core.windows.net
What Happens When Base Is Added To Water at Jose Oliver blog What Happens If You Add A Base To An Acid when you add an acid or base to the buffer, the buffer components react to neutralize the added substance. The product of such a. Moreover, many of the substances we encounter in our homes,. if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. acids are substances. What Happens If You Add A Base To An Acid.
From www.sigmaaldrich.cn
Acid and Base Chart — Table of Acids & Bases What Happens If You Add A Base To An Acid Bases ionize for form hydroxy ions in solution. An acid and a base are like chemical opposites. How do acids and bases neutralize one another (or cancel each other out)? If a base is added to an. acids are substances that ionize to form hydrogen ions in solution. the added \ (hcl\) (a strong acid) or \ (naoh\). What Happens If You Add A Base To An Acid.
From studylib.net
AcidBase Chemistry What Happens If You Add A Base To An Acid if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. acids are substances that ionize to form hydrogen ions in solution. How do acids and bases neutralize one another (or cancel each other out)? Bases ionize for form hydroxy ions in solution. when you add an acid. What Happens If You Add A Base To An Acid.
From www.worksheetsplanet.com
Acid and Bases Differences What Happens If You Add A Base To An Acid adding a base decreases the concentration of h 3 o + ions in the solution. How do acids and bases neutralize one another (or cancel each other out)? the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. Bases ionize for form hydroxy ions in solution.. What Happens If You Add A Base To An Acid.
From mavink.com
What Is Acid Base Reaction What Happens If You Add A Base To An Acid Bases ionize for form hydroxy ions in solution. Moreover, many of the substances we encounter in our homes,. An acid and a base are like chemical opposites. adding a base decreases the concentration of h 3 o + ions in the solution. How do acids and bases neutralize one another (or cancel each other out)? acids are substances. What Happens If You Add A Base To An Acid.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive What Happens If You Add A Base To An Acid adding a base decreases the concentration of h 3 o + ions in the solution. An acid and a base are like chemical opposites. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. How do acids and bases neutralize one another (or cancel each other. What Happens If You Add A Base To An Acid.
From www.sliderbase.com
AcidBase Reactions Presentation Chemistry What Happens If You Add A Base To An Acid If a base is added to an. acids are substances that ionize to form hydrogen ions in solution. if you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other. This keeps the ph relatively stable. Moreover, many of the substances we encounter in our homes,. An acid and a. What Happens If You Add A Base To An Acid.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID What Happens If You Add A Base To An Acid acids are substances that ionize to form hydrogen ions in solution. Bases ionize for form hydroxy ions in solution. The product of such a. adding a base decreases the concentration of h 3 o + ions in the solution. when you add an acid or base to the buffer, the buffer components react to neutralize the added. What Happens If You Add A Base To An Acid.
From www.slideserve.com
PPT What happens when you put an acid and a base together? PowerPoint What Happens If You Add A Base To An Acid Moreover, many of the substances we encounter in our homes,. How do acids and bases neutralize one another (or cancel each other out)? The product of such a. the added \ (hcl\) (a strong acid) or \ (naoh\) (a strong base) will react completely with formate (a weak base) or. If a base is added to an. Bases ionize. What Happens If You Add A Base To An Acid.