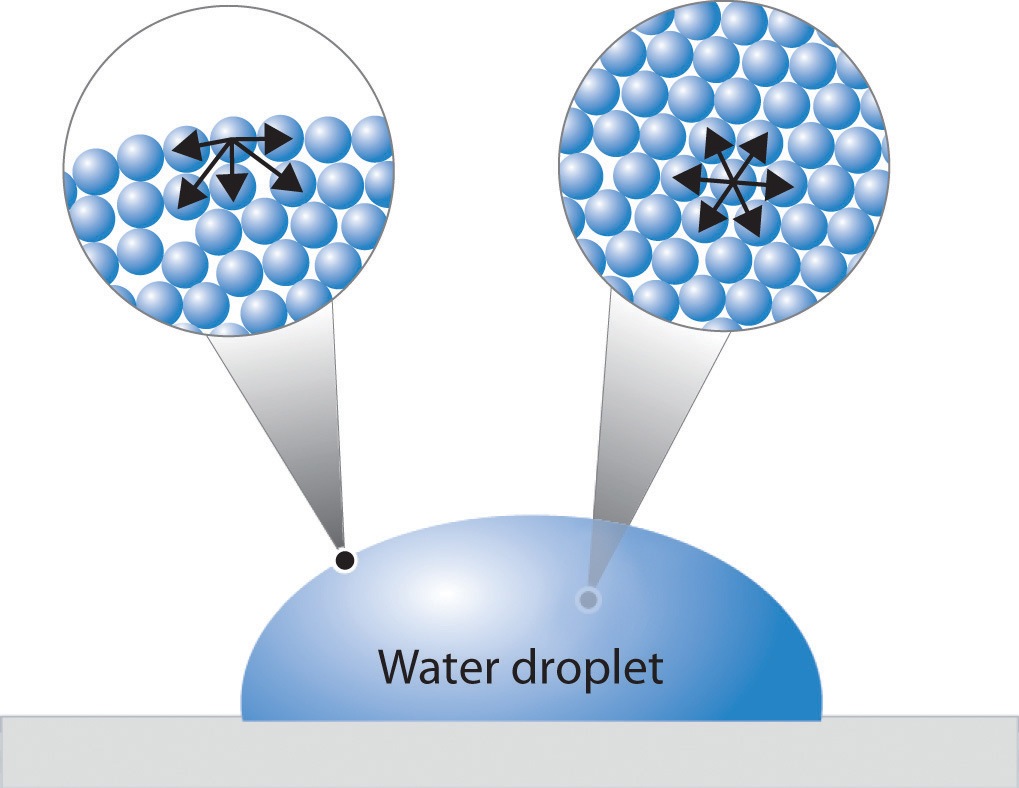
Water's Surface Tension Is . In comparison, organic liquids, such as benzene. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. It would take a force of 72 dynes to break a surface film of water 1 cm long. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water consists of one oxygen atom flanked by two hydrogen. The surface tension of water decreases. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of water is 72 dynes/cm at 25°c. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is a manifestation of the.
from chem.libretexts.org
water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is a manifestation of the. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. In comparison, organic liquids, such as benzene. It would take a force of 72 dynes to break a surface film of water 1 cm long. next to mercury, water has the highest surface tension of all commonly occurring liquids. The surface tension of water decreases. the surface tension of water is 72 dynes/cm at 25°c. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
Surface Tension Chemistry LibreTexts
Water's Surface Tension Is water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water consists of one oxygen atom flanked by two hydrogen. The surface tension of water decreases. Surface tension is a manifestation of the. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). It would take a force of 72 dynes to break a surface film of water 1 cm long. the surface tension of water is 72 dynes/cm at 25°c. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene.
From exolnazbp.blob.core.windows.net
Water Surface Tension With Temperature at Amber Holmes blog Water's Surface Tension Is water has high surface tension, which can be explained by its polarity and hydrogen bonding. the surface tension of water is 72 dynes/cm at 25°c. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. water has a surface tension of 0.07275. Water's Surface Tension Is.
From www.thoughtco.com
Surface Tension Definition in Chemistry Water's Surface Tension Is water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). next to mercury, water has. Water's Surface Tension Is.
From cektxihx.blob.core.windows.net
Water Surface Tension En Francais at Fletcher Levesque blog Water's Surface Tension Is In comparison, organic liquids, such as benzene. The surface tension of water decreases. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. water has a surface tension. Water's Surface Tension Is.
From ar.inspiredpencil.com
Surface Tension Water Water's Surface Tension Is water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene. The surface tension of water decreases. It would take a force of 72 dynes to break a surface film of water 1 cm long. Water consists of one oxygen atom flanked by two hydrogen. water. Water's Surface Tension Is.
From www.usgs.gov
Surface Tension and Water U.S. Geological Survey Water's Surface Tension Is surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water consists of one oxygen atom flanked by two hydrogen. It would take a force of 72 dynes. Water's Surface Tension Is.
From cekftzsc.blob.core.windows.net
Surface Tension Water Examples at Doreen Rech blog Water's Surface Tension Is Surface tension is a manifestation of the. Water consists of one oxygen atom flanked by two hydrogen. the surface tension of water is 72 dynes/cm at 25°c. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has high surface tension, which can be explained by. Water's Surface Tension Is.
From www.biolinscientific.com
Surface tension of water Why is it so high? Water's Surface Tension Is The surface tension of water decreases. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. In comparison, organic liquids, such as benzene. water has a surface tension of 0.07275. Water's Surface Tension Is.
From cekftzsc.blob.core.windows.net
Surface Tension Water Examples at Doreen Rech blog Water's Surface Tension Is the surface tension of water is 72 dynes/cm at 25°c. Surface tension is a manifestation of the. In comparison, organic liquids, such as benzene. Water consists of one oxygen atom flanked by two hydrogen. water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule. Water's Surface Tension Is.
From www.biolinscientific.com
Surface tension of water Why is it so high? Water's Surface Tension Is Surface tension is a manifestation of the. It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. surface tension is the energy, or work, required to. Water's Surface Tension Is.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Water's Surface Tension Is surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. the surface tension of water is 72 dynes/cm at 25°c. Surface tension is a manifestation of the. It would take a force of 72 dynes to break a surface film of water 1 cm. Water's Surface Tension Is.
From www.biolinscientific.com
Why is surface tension important? Water's Surface Tension Is water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of water is 72 dynes/cm at 25°c. The surface tension of water decreases. next to mercury, water has. Water's Surface Tension Is.
From circuitenginemeseta101.z22.web.core.windows.net
Diagram Of Surface Tension Water's Surface Tension Is It would take a force of 72 dynes to break a surface film of water 1 cm long. Water consists of one oxygen atom flanked by two hydrogen. Surface tension is a manifestation of the. The surface tension of water decreases. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension. Water's Surface Tension Is.
From sciencewithkids.com
Water Surface Tension Experiment Science with Water's Surface Tension Is Water consists of one oxygen atom flanked by two hydrogen. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Surface tension is a manifestation of the. the surface tension of water is 72 dynes/cm at 25°c. surface tension is the energy, or work, required to increase the surface area of a. Water's Surface Tension Is.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Water's Surface Tension Is Surface tension is a manifestation of the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. Water consists of one oxygen atom flanked. Water's Surface Tension Is.
From dxoxudzok.blob.core.windows.net
Surface Tension Of Water Pas at Debra Santiago blog Water's Surface Tension Is surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. next to mercury, water has the highest surface tension of all commonly occurring liquids. It would take a force of 72 dynes to break a surface film of water 1 cm long. Surface tension is a manifestation of. Water's Surface Tension Is.
From funsizephysics.com
What is Surface Tension? FunsizePhysics Water's Surface Tension Is surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. It would take a force of 72 dynes to break a surface film of water 1 cm long. the surface tension of water is 72 dynes/cm at 25°c. surface tension is the energy, or work, required to. Water's Surface Tension Is.
From www.australianenvironmentaleducation.com.au
Water is found everywhere on earth, so why is it important? Water's Surface Tension Is surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. next to mercury, water has the highest surface tension of all commonly occurring liquids. Water consists of. Water's Surface Tension Is.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance Water's Surface Tension Is the surface tension of water is 72 dynes/cm at 25°c. water has high surface tension, which can be explained by its polarity and hydrogen bonding. next to mercury, water has the highest surface tension of all commonly occurring liquids. In comparison, organic liquids, such as benzene. The surface tension of water decreases. surface tension is the. Water's Surface Tension Is.
From www.youtube.com
Surface Tension of Water Explained YouTube Water's Surface Tension Is surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. In comparison, organic liquids, such as benzene. Surface tension is a manifestation of the. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.. Water's Surface Tension Is.
From manualpartmechlin123.z5.web.core.windows.net
Dimension Of Tension And Surface Tension Water's Surface Tension Is It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as benzene. The surface tension of water decreases. water has high surface tension, which can. Water's Surface Tension Is.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments Water's Surface Tension Is water has high surface tension, which can be explained by its polarity and hydrogen bonding. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. It would. Water's Surface Tension Is.
From mavink.com
Water Molecules Surface Tension Water's Surface Tension Is It would take a force of 72 dynes to break a surface film of water 1 cm long. water has high surface tension, which can be explained by its polarity and hydrogen bonding. In comparison, organic liquids, such as benzene. surface tension is the energy, or work, required to increase the surface area of a liquid due to. Water's Surface Tension Is.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download Water's Surface Tension Is the surface tension of water is 72 dynes/cm at 25°c. Water consists of one oxygen atom flanked by two hydrogen. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is a manifestation of the. The surface tension of water decreases. water has a surface. Water's Surface Tension Is.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Water's Surface Tension Is next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension in water might be. Water's Surface Tension Is.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Water's Surface Tension Is In comparison, organic liquids, such as benzene. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Water consists of one oxygen atom flanked by two hydrogen. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has high surface tension, which. Water's Surface Tension Is.
From cekftzsc.blob.core.windows.net
Surface Tension Water Examples at Doreen Rech blog Water's Surface Tension Is surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water has high surface tension, which can be explained by its polarity and. Water's Surface Tension Is.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Water's Surface Tension Is water has high surface tension, which can be explained by its polarity and hydrogen bonding. next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. Surface tension is a. Water's Surface Tension Is.
From www.greenkidcrafts.com
Water Surface Tension Experiment for Kids Green Kid Crafts Water's Surface Tension Is Surface tension is a manifestation of the. surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. the surface tension of water is. Water's Surface Tension Is.
From byjus.com
Explain the surface tension phenomenon with examples. Water's Surface Tension Is The surface tension of water decreases. the surface tension of water is 72 dynes/cm at 25°c. It would take a force of 72 dynes to break a surface film of water 1 cm long. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is. Water's Surface Tension Is.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse Water's Surface Tension Is Surface tension is a manifestation of the. It would take a force of 72 dynes to break a surface film of water 1 cm long. water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen. water has a surface tension of 0.07275 joule. Water's Surface Tension Is.
From pixels.com
Surface Tension Photograph by Dr. John Brackenbury/science Photo Library Water's Surface Tension Is Water consists of one oxygen atom flanked by two hydrogen. Surface tension is a manifestation of the. next to mercury, water has the highest surface tension of all commonly occurring liquids. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). surface tension in water might be good at performing tricks,. Water's Surface Tension Is.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog Water's Surface Tension Is water has high surface tension, which can be explained by its polarity and hydrogen bonding. It would take a force of 72 dynes to break a surface film of water 1 cm long. Water consists of one oxygen atom flanked by two hydrogen. surface tension is the energy, or work, required to increase the surface area of a. Water's Surface Tension Is.
From www.youtube.com
Water's surface tension physics experiment YouTube Water's Surface Tension Is surface tension in water might be good at performing tricks, such as being able to float a paper clip on its surface, but surface. It would take a force of 72 dynes to break a surface film of water 1 cm long. The surface tension of water decreases. next to mercury, water has the highest surface tension of. Water's Surface Tension Is.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 Water's Surface Tension Is In comparison, organic liquids, such as benzene. Water consists of one oxygen atom flanked by two hydrogen. the surface tension of water is 72 dynes/cm at 25°c. water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). next to mercury, water has the highest surface tension of all commonly occurring liquids.. Water's Surface Tension Is.
From www.science-sparks.com
Surface Tension of Water Science Experiments for Kids Water's Surface Tension Is Water consists of one oxygen atom flanked by two hydrogen. water has high surface tension, which can be explained by its polarity and hydrogen bonding. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. In comparison, organic liquids, such as benzene. surface tension in water might. Water's Surface Tension Is.