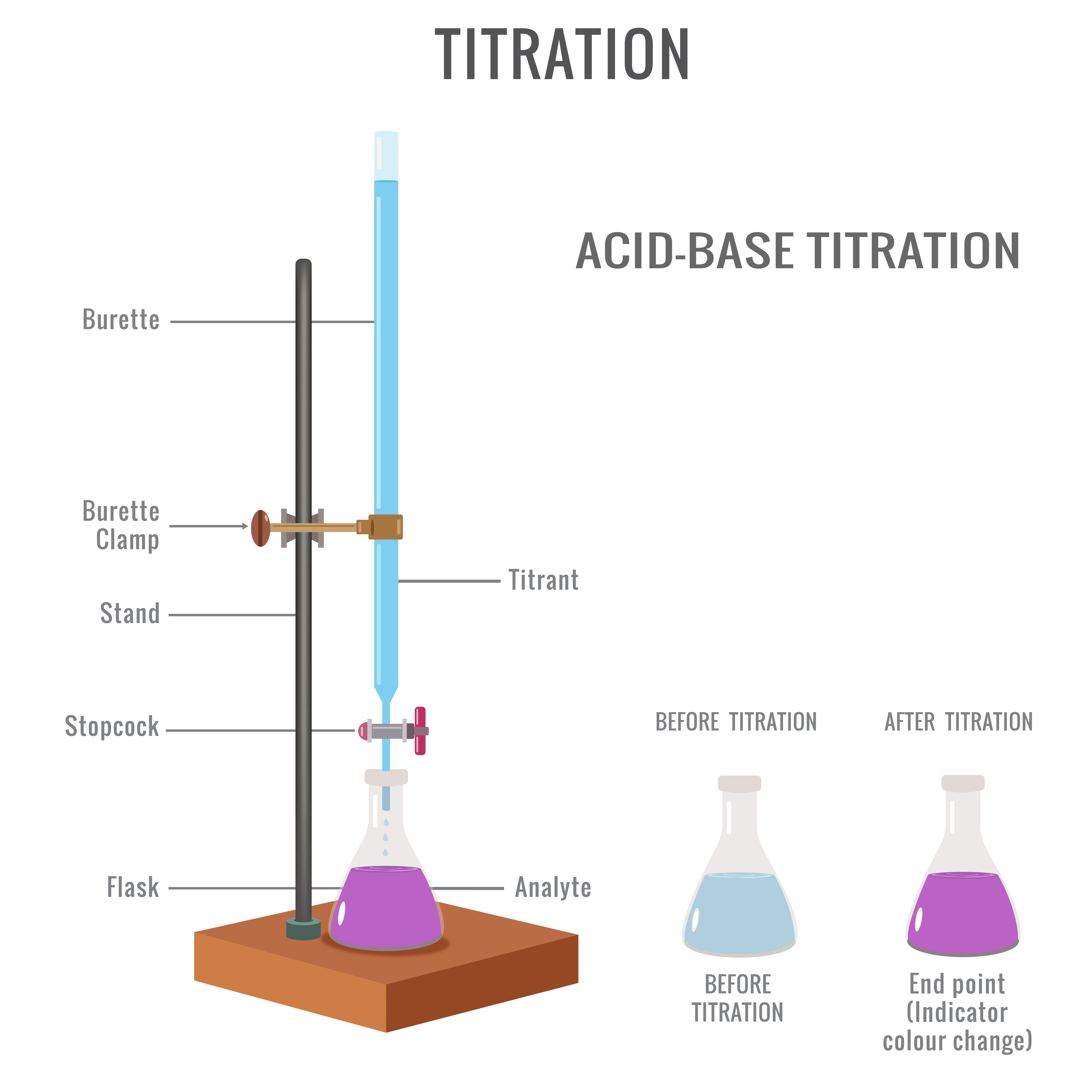
Diagram Of A Titration Setup . Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. In an indicator based titration you add another. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Are an experimental method🧪 used to determine the unknown. There are two basic types of acid base titrations, indicator and potentiometric.
from www.calpaclab.com
Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. In an indicator based titration you add another. Are an experimental method🧪 used to determine the unknown. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. There are two basic types of acid base titrations, indicator and potentiometric.
The Science of Titration Analysis CP Lab Safety
Diagram Of A Titration Setup Are an experimental method🧪 used to determine the unknown. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Are an experimental method🧪 used to determine the unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or.
From www.shutterstock.com
Titration Setup Educational Chemistry Diagram Stock Illustration 2189329325 Shutterstock Diagram Of A Titration Setup There are two basic types of acid base titrations, indicator and potentiometric. Are an experimental method🧪 used to determine the unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Be able to determine the k a or. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup In an indicator based titration you add another. Are an experimental method🧪 used to determine the unknown. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup There are two basic types of acid base titrations, indicator and potentiometric. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. In an indicator based titration you add another. Be able to determine the k a or k b from ph data associated with the titration of a. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. A titration. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Are an experimental method🧪 used to determine the unknown. In an indicator based titration you add another. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is a volumetric. Diagram Of A Titration Setup.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Diagram Of A Titration Setup In an indicator based titration you add another. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Be able to determine the k a or k b from ph data associated with the titration of a weak acid. Diagram Of A Titration Setup.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Diagram Of A Titration Setup Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Diagram Of A Titration Setup.
From www.vecteezy.com
Acid base titration experiment and phases of color change during titration 23452927 Vector Art Diagram Of A Titration Setup Are an experimental method🧪 used to determine the unknown. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. There are two basic types of acid base titrations, indicator and potentiometric. Be able to determine the k a or k b from ph data associated with the titration of a. Diagram Of A Titration Setup.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration TutorMyself Chemistry Diagram Of A Titration Setup Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. In an indicator based titration you add another. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant). Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration is. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Are an experimental method🧪 used to determine the unknown. In an indicator based titration you add another. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. Titration. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup There are two basic types of acid base titrations, indicator and potentiometric. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. In an indicator based titration you add another. Are an experimental method🧪 used to determine the unknown.. Diagram Of A Titration Setup.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Diagram Of A Titration Setup Are an experimental method🧪 used to determine the unknown. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. Determine the concentration of analyte present, as well as. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Are an experimental method🧪 used to determine the unknown. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that. Diagram Of A Titration Setup.
From www.calpaclab.com
The Science of Titration Analysis CP Lab Safety Diagram Of A Titration Setup There are two basic types of acid base titrations, indicator and potentiometric. Are an experimental method🧪 used to determine the unknown. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. Diagram Of A Titration Setup.
From www.youtube.com
Titration tube How to draw well labelled Titration Tube Titration Diagram Chemistry Diagram Of A Titration Setup There are two basic types of acid base titrations, indicator and potentiometric. Are an experimental method🧪 used to determine the unknown. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. 1.1 a titration involves measuring the exact volume. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. There are two basic types of acid base titrations, indicator and potentiometric. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is a volumetric technique. Diagram Of A Titration Setup.
From collegedunia.com
Volumetric Analysis Titration, Types, Principle & Procedure Diagram Of A Titration Setup Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. In an indicator based titration you add another. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Are an experimental method🧪 used. Diagram Of A Titration Setup.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Point Diagram Of A Titration Setup A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Determine the concentration of analyte present, as. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. In an indicator based titration you add another. There are two basic types of acid base titrations,. Diagram Of A Titration Setup.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Diagram Of A Titration Setup Are an experimental method🧪 used to determine the unknown. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. 1.1 a titration involves measuring the exact volume of a. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup There are two basic types of acid base titrations, indicator and potentiometric. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup There are two basic types of acid base titrations, indicator and potentiometric. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. In an indicator based titration you add another. Are an experimental method🧪. Diagram Of A Titration Setup.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Diagram Of A Titration Setup A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Are an experimental method🧪 used to determine the unknown. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another.. Diagram Of A Titration Setup.
From theedge.com.hk
Chemistry How To Titration The Edge Diagram Of A Titration Setup In an indicator based titration you add another. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Diagram Of A Titration Setup.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Diagram Of A Titration Setup Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. There are two basic types of acid base titrations, indicator and potentiometric. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. Are an experimental method🧪 used to determine the unknown. Be able to. Diagram Of A Titration Setup.
From www.tes.com
Titration Edexcel 91 Separate (Triple) Science Teaching Resources Diagram Of A Titration Setup Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup Are an experimental method🧪 used to determine the unknown. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point. Diagram Of A Titration Setup.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Diagram Of A Titration Setup In an indicator based titration you add another. Are an experimental method🧪 used to determine the unknown. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Titration is a quantitative technique that uses. Diagram Of A Titration Setup.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic15_acidsalkalisandtitrations_004_titrationapparatussetup Diagram Of A Titration Setup Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. In an indicator based titration you add another. There are two basic types of acid base titrations,. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup There are two basic types of acid base titrations, indicator and potentiometric. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Are an experimental method🧪 used to determine the unknown. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. 1.1. Diagram Of A Titration Setup.
From ar.inspiredpencil.com
Titration Setup Diagram Diagram Of A Titration Setup 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. Diagram Of A Titration Setup.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Diagram Of A Titration Setup Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is a quantitative technique that uses a solution of known concentration to react completely with an unknown to determine the. There. Diagram Of A Titration Setup.