What Happens When You Mix Water And Ethanol . Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Ethanol molecules are much smaller in size. These two liquids form a homogeneous mixture due to their similar molecular. When you mix isopropyl alcohol and water, something fascinating occurs. If not, then the rest depends. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. They are both polar substances and. What happens when you mix ethyl alcohol and water? Ethanol molecules are smaller than water molecules, so when the two liquids. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen.
from www.chegg.com
Ethanol molecules are much smaller in size. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. These two liquids form a homogeneous mixture due to their similar molecular. They are both polar substances and. What happens when you mix ethyl alcohol and water? Water and alcohol, when mixed together, will not form separate layers due to their miscibility. When you mix isopropyl alcohol and water, something fascinating occurs. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. If not, then the rest depends.
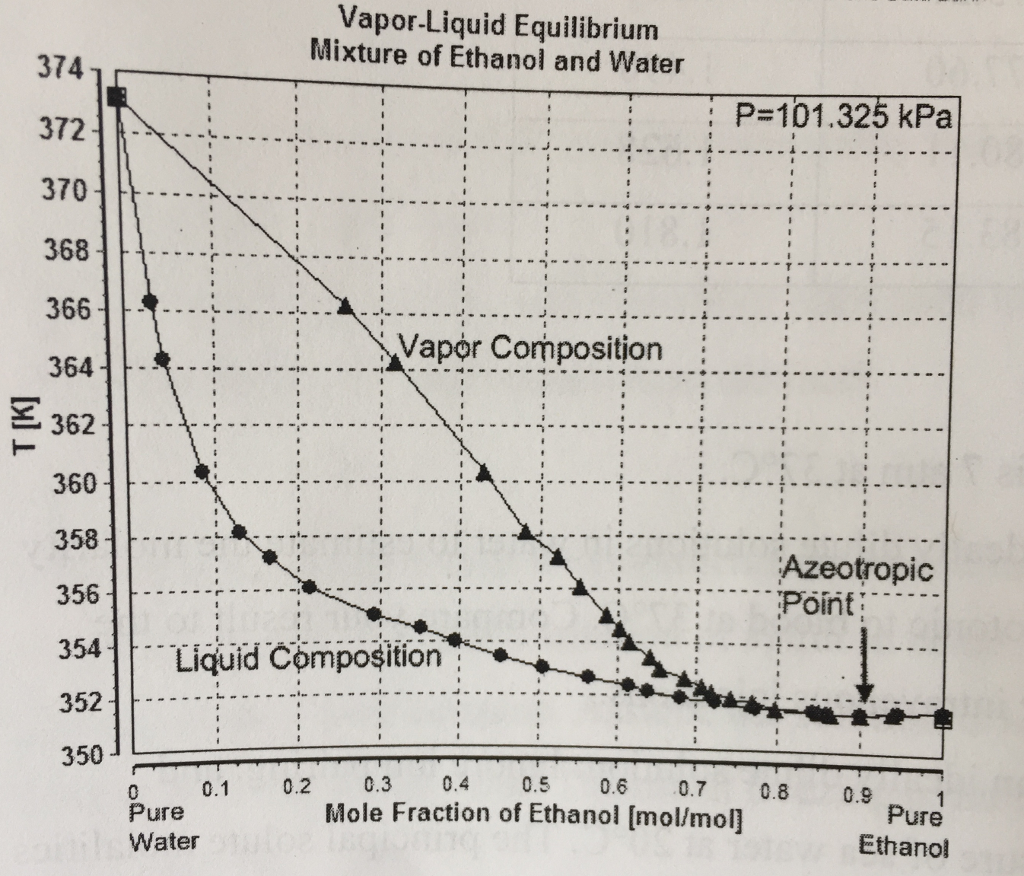
Solved VaporLiquid Equilibrium Mixture of Ethanol and Water
What Happens When You Mix Water And Ethanol Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. If not, then the rest depends. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. These two liquids form a homogeneous mixture due to their similar molecular. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. Ethanol molecules are much smaller in size. Ethanol molecules are smaller than water molecules, so when the two liquids. They are both polar substances and. What happens when you mix ethyl alcohol and water? When you mix isopropyl alcohol and water, something fascinating occurs. Water and alcohol, when mixed together, will not form separate layers due to their miscibility.
From hubpages.com
Chemistry of waterethanol mixture HubPages What Happens When You Mix Water And Ethanol Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Ethanol molecules are smaller than water molecules, so when the two liquids. If not, then the rest depends. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. They are both polar substances and. Water and. What Happens When You Mix Water And Ethanol.
From wiringpictures.net
Exploring the Txy Diagram for Ethanol and Water What Happens When You Mix Water And Ethanol Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. They are both polar substances and. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. Ethanol molecules are much smaller. What Happens When You Mix Water And Ethanol.
From exojrfljr.blob.core.windows.net
What Happens When You Mix Rubbing Alcohol And Water at Guillermo Petit blog What Happens When You Mix Water And Ethanol If not, then the rest depends. What happens when you mix ethyl alcohol and water? Ethanol molecules are much smaller in size. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. Ethanol molecules are smaller than water molecules,. What Happens When You Mix Water And Ethanol.
From www.slideserve.com
PPT Mixing ethanol and water lab… PowerPoint Presentation, free download ID2676609 What Happens When You Mix Water And Ethanol If not, then the rest depends. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. Ethanol. What Happens When You Mix Water And Ethanol.
From www.youtube.com
DISTILLATION OF WATER AND ETHANOL YouTube What Happens When You Mix Water And Ethanol Water and alcohol, when mixed together, will not form separate layers due to their miscibility. These two liquids form a homogeneous mixture due to their similar molecular. Ethanol molecules are much smaller in size. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. When you mix isopropyl alcohol and. What Happens When You Mix Water And Ethanol.
From www.researchgate.net
Vapourliquid equilibrium of ethanolwater showing distillation steps... Download Scientific What Happens When You Mix Water And Ethanol These two liquids form a homogeneous mixture due to their similar molecular. What happens when you mix ethyl alcohol and water? When you mix isopropyl alcohol and water, something fascinating occurs. If not, then the rest depends. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. Could someone explain why volume contraction occurs. What Happens When You Mix Water And Ethanol.
From byjus.com
What type of mixture is Ethanol and water What Happens When You Mix Water And Ethanol Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. What happens when you mix ethyl alcohol and water?. What Happens When You Mix Water And Ethanol.
From www.chegg.com
Solved VaporLiquid Equilibrium Mixture of Ethanol and Water What Happens When You Mix Water And Ethanol Ethanol molecules are much smaller in size. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. What happens when you mix ethyl alcohol and water? Water and alcohol, when mixed together, will not form separate layers due to their miscibility. If not, then the rest depends. These two liquids form a. What Happens When You Mix Water And Ethanol.
From www.sciencephoto.com
Ethanol and water Stock Image C036/3926 Science Photo Library What Happens When You Mix Water And Ethanol Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. These two liquids form a homogeneous mixture due to their similar molecular. They are both polar substances and. Ethanol molecules are smaller than water molecules, so when the two liquids. Could someone explain why volume contraction occurs when you mix an alcohol such as. What Happens When You Mix Water And Ethanol.
From exojrfljr.blob.core.windows.net
What Happens When You Mix Rubbing Alcohol And Water at Guillermo Petit blog What Happens When You Mix Water And Ethanol Ethanol molecules are much smaller in size. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol. What Happens When You Mix Water And Ethanol.
From www.fabhow.com
How to Use Rubbing Alcohol for Health and Beauty Fab How What Happens When You Mix Water And Ethanol Ethanol molecules are much smaller in size. These two liquids form a homogeneous mixture due to their similar molecular. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. What happens when you mix ethyl alcohol and water? If not, then the rest depends. Could someone explain why volume contraction occurs when. What Happens When You Mix Water And Ethanol.
From w20.b2m.cz
Uma Solução é Formada Por 115g De Etanol EDUCA What Happens When You Mix Water And Ethanol Water and alcohol, when mixed together, will not form separate layers due to their miscibility. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. They are both polar substances and. Ethanol molecules are smaller than water molecules, so when the two liquids. If not, then the rest depends. When. What Happens When You Mix Water And Ethanol.
From www.sciencephoto.com
Water and ethanol Stock Image C044/8781 Science Photo Library What Happens When You Mix Water And Ethanol Water and alcohol, when mixed together, will not form separate layers due to their miscibility. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. If not, then the rest depends. What. What Happens When You Mix Water And Ethanol.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6540865 What Happens When You Mix Water And Ethanol Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. These two liquids form a homogeneous mixture due to their similar molecular. If not, then the rest depends. What happens when you. What Happens When You Mix Water And Ethanol.
From www.youtube.com
Mixing water and alcohol YouTube What Happens When You Mix Water And Ethanol What happens when you mix ethyl alcohol and water? Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. These two liquids form a homogeneous mixture due to their similar molecular. They are both polar substances and. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water. What Happens When You Mix Water And Ethanol.
From www.slideserve.com
PPT Ch. 11 Liquids, Solids, and Intermolecular Forces PowerPoint Presentation ID1177746 What Happens When You Mix Water And Ethanol Ethanol molecules are smaller than water molecules, so when the two liquids. When you mix isopropyl alcohol and water, something fascinating occurs. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. What happens when you mix ethyl alcohol and water? These two liquids form a homogeneous mixture due to their similar. What Happens When You Mix Water And Ethanol.
From mavink.com
Ethanol Water Distillation What Happens When You Mix Water And Ethanol Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. What happens when you mix ethyl alcohol and water? If not, then the rest depends. Could someone explain why volume contraction occurs when you mix an. What Happens When You Mix Water And Ethanol.
From www.slideserve.com
PPT Why Don't Alcohol and Water Mix Very Well? “The molecular structure of Alcoholwater What Happens When You Mix Water And Ethanol Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. What happens when you mix ethyl alcohol and water? These two liquids form a homogeneous mixture due to their similar molecular. Ethanol. What Happens When You Mix Water And Ethanol.
From www.researchgate.net
Flashpoint of liquid water/ethanol mixtures Download Scientific Diagram What Happens When You Mix Water And Ethanol When you mix isopropyl alcohol and water, something fascinating occurs. If not, then the rest depends. Ethanol molecules are much smaller in size. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. These two liquids form a homogeneous mixture due to their similar molecular. Water and alcohol, when mixed. What Happens When You Mix Water And Ethanol.
From www.youtube.com
Mixing water/water and ethanol/ethanol YouTube What Happens When You Mix Water And Ethanol When you mix isopropyl alcohol and water, something fascinating occurs. Ethanol molecules are much smaller in size. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. They are both polar substances and. What happens when you mix ethyl alcohol and water? Ethanol molecules are smaller than water molecules, so. What Happens When You Mix Water And Ethanol.
From www.researchgate.net
Evaporation of water, ethanol, and water/ethanol mixtures with an... Download Scientific Diagram What Happens When You Mix Water And Ethanol Ethanol molecules are smaller than water molecules, so when the two liquids. If not, then the rest depends. They are both polar substances and. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. These two liquids. What Happens When You Mix Water And Ethanol.
From www.researchgate.net
(a) Mass density and (b) viscosity of waterethanol mixtures, as a... Download Scientific Diagram What Happens When You Mix Water And Ethanol If not, then the rest depends. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. They are both polar substances and. When you mix isopropyl alcohol and water, something fascinating occurs. These two liquids form a homogeneous mixture due to their similar molecular. Could someone explain why volume contraction occurs when. What Happens When You Mix Water And Ethanol.
From www.chegg.com
Solved Q.2 A mixture of ethanol (ethyl alcohol) and water What Happens When You Mix Water And Ethanol Water and alcohol, when mixed together, will not form separate layers due to their miscibility. These two liquids form a homogeneous mixture due to their similar molecular. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. What happens when you mix ethyl alcohol and water? Ethanol molecules are smaller. What Happens When You Mix Water And Ethanol.
From www.researchgate.net
Distillation of ethanolwater mixture. The most common processes are... Download Scientific What Happens When You Mix Water And Ethanol They are both polar substances and. What happens when you mix ethyl alcohol and water? Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. These two liquids form a homogeneous mixture due to their similar molecular.. What Happens When You Mix Water And Ethanol.
From www.slideserve.com
PPT Mixing ethanol and water lab… PowerPoint Presentation, free download ID2676609 What Happens When You Mix Water And Ethanol What happens when you mix ethyl alcohol and water? Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. These two liquids form a homogeneous mixture due to their similar molecular. When you mix isopropyl alcohol and water, something fascinating occurs. If not, then the rest depends. Ethanol molecules are. What Happens When You Mix Water And Ethanol.
From byjus.com
Name the process you would use to separate a mixture of water and alcohol. What Happens When You Mix Water And Ethanol Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. When you mix isopropyl alcohol and water, something fascinating occurs. Ethanol molecules are much smaller in size. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. Ethanol and water are mixed. What Happens When You Mix Water And Ethanol.
From www.youtube.com
Mixing ethanol and water YouTube What Happens When You Mix Water And Ethanol Ethanol molecules are smaller than water molecules, so when the two liquids. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. They are both polar substances and. What happens when you. What Happens When You Mix Water And Ethanol.
From delilah-has-sheppard.blogspot.com
Gasoline and Water Do Not Mix Because DelilahhasSheppard What Happens When You Mix Water And Ethanol If not, then the rest depends. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. What happens when you mix ethyl alcohol and water? Ethanol molecules are smaller than water molecules, so when the two liquids. These two liquids form a homogeneous mixture due to their similar molecular. When you mix isopropyl alcohol. What Happens When You Mix Water And Ethanol.
From www.youtube.com
A solution is prepared by mixing ethanol and water. The mole fraction of ethanol in the mixture What Happens When You Mix Water And Ethanol They are both polar substances and. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. Ethanol molecules are smaller than water molecules, so when the two liquids. Ethanol molecules are much smaller in size. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. Water. What Happens When You Mix Water And Ethanol.
From advchemyellow1.blogspot.com
Consumer Chemistry Lets Mix Oil and Water Part 2 Thermodynamics What Happens When You Mix Water And Ethanol What happens when you mix ethyl alcohol and water? Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. These two liquids form a homogeneous mixture due to their similar molecular. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. They are both polar substances and.. What Happens When You Mix Water And Ethanol.
From recoveryranger.com
How Do You Separate Alcohol And Water? Recovery Ranger What Happens When You Mix Water And Ethanol When you mix isopropyl alcohol and water, something fascinating occurs. If not, then the rest depends. Water and alcohol, when mixed together, will not form separate layers due to their miscibility. Could someone explain why volume contraction occurs when you mix an alcohol such as ethanol with water in relation hydrogen. They are both polar substances and. What happens when. What Happens When You Mix Water And Ethanol.
From www.sciencephoto.com
Water and ethanol Stock Image C044/8783 Science Photo Library What Happens When You Mix Water And Ethanol Water and alcohol, when mixed together, will not form separate layers due to their miscibility. What happens when you mix ethyl alcohol and water? When you mix isopropyl alcohol and water, something fascinating occurs. These two liquids form a homogeneous mixture due to their similar molecular. They are both polar substances and. If not, then the rest depends. Ethanol and. What Happens When You Mix Water And Ethanol.
From www.slideserve.com
PPT Mixing ethanol and water lab… PowerPoint Presentation, free download ID2676609 What Happens When You Mix Water And Ethanol Water and alcohol, when mixed together, will not form separate layers due to their miscibility. Ethanol molecules are much smaller in size. What happens when you mix ethyl alcohol and water? If not, then the rest depends. Ethanol and water are mixed in volumetric glassware, showing a volume decrease and a temperature increase. These two liquids form a homogeneous mixture. What Happens When You Mix Water And Ethanol.
From www.researchgate.net
(a) C G in waterethanol mixture without surfactant and the surface... Download Scientific Diagram What Happens When You Mix Water And Ethanol What happens when you mix ethyl alcohol and water? Ethanol molecules are smaller than water molecules, so when the two liquids. When you mix isopropyl alcohol and water, something fascinating occurs. Ethanol molecules are smaller than water molecules, so when the two liquids are mixed together the ethanol falls. These two liquids form a homogeneous mixture due to their similar. What Happens When You Mix Water And Ethanol.
From www.youtube.com
250+250=490 missing volume when mixing water and ethanol YouTube What Happens When You Mix Water And Ethanol If not, then the rest depends. Ethanol molecules are smaller than water molecules, so when the two liquids. When you mix isopropyl alcohol and water, something fascinating occurs. They are both polar substances and. These two liquids form a homogeneous mixture due to their similar molecular. What happens when you mix ethyl alcohol and water? Water and alcohol, when mixed. What Happens When You Mix Water And Ethanol.