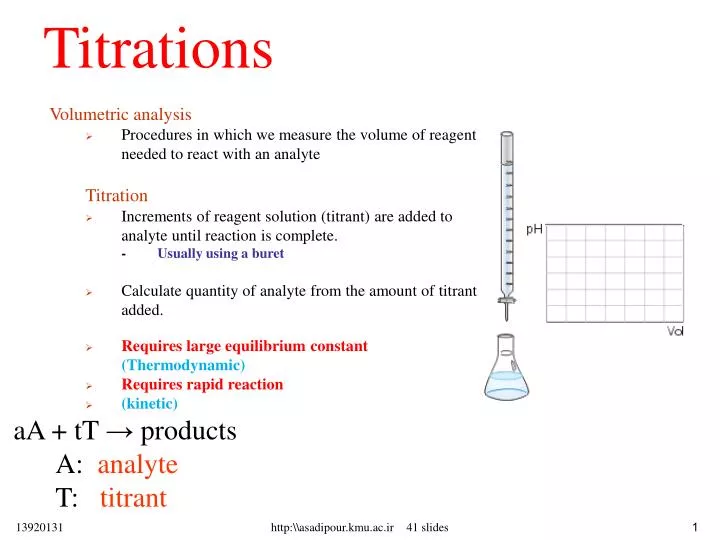
Titration Analysis Function . Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. This is what is known as titration. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. For example, you can use it to find the concentration of a. Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution.
from www.slideserve.com
Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For example, you can use it to find the concentration of a. This is what is known as titration.
PPT Titrations PowerPoint Presentation, free download ID2145156
Titration Analysis Function This is what is known as titration. For example, you can use it to find the concentration of a. Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. This is what is known as titration.
From saylordotorg.github.io
AcidBase Titrations Titration Analysis Function Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. This is what is known as titration. Titration is the slow addition of one solution of a known. Titration Analysis Function.
From mungfali.com
Titration Graph Titration Analysis Function Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. This is what is known as titration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. A quantitative chemical analysis in which a defined amount of titrant reacts. Titration Analysis Function.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Presentation ID1995345 Titration Analysis Function For example, you can use it to find the concentration of a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured. Titration Analysis Function.
From mungfali.com
Titration Process Titration Analysis Function Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. This is what is known as titration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. For example, you can use it to find the concentration of a.. Titration Analysis Function.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Analysis Function Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound. Titration Analysis Function.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Analysis Function A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. For example, you can use it to find the concentration of a. This is what is known as. Titration Analysis Function.
From knowledge.carolina.com
Titrations Techniques and Calculations Carolina Knowledge Center Titration Analysis Function A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. For example, you can use it to find the concentration of a. This is what is known as titration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration Analysis Function.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations, Examples, Solution Stoichiometry Titration Analysis Function This is what is known as titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A quantitative chemical analysis in which a defined amount of. Titration Analysis Function.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145156 Titration Analysis Function A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. This is what is known as titration. For example, you can use it to find the concentration of. Titration Analysis Function.
From www.slideserve.com
PPT CHAPTER 5 Volumetric Analysis PowerPoint Presentation, free download ID5728705 Titration Analysis Function A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. This is what is known as titration. For example, you can use it to find the concentration of a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Titration Analysis Function.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Analysis Function A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. Titration is. Titration Analysis Function.
From www.youtube.com
Excel Tutorial 2 Titration Analysis YouTube Titration Analysis Function Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. For example, you can use it to find the concentration of a. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. This is what is. Titration Analysis Function.
From chem.libretexts.org
Quantitative Analysis Nuts and Bolts Chemistry LibreTexts Titration Analysis Function Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. Titration is a way of measuring the concentration of something, usually the concentration of a substance. Titration Analysis Function.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Analysis Function Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. This is what is known as titration. For example, you can use it to find the concentration of. Titration Analysis Function.
From springofchemistry.blogspot.com
Spring Of Chemistry Diagram for titration Titration Analysis Function A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another. Titration Analysis Function.
From mavink.com
Titration Reaction Titration Analysis Function Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. A “classical” redox titration is one in which a titrant of a known concentration is prepared and. Titration Analysis Function.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Analysis Function For example, you can use it to find the concentration of a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. Dissolving the analyte and making. Titration Analysis Function.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Analysis Function Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. Dissolving the analyte and making it react with another species in. Titration Analysis Function.
From www.slideserve.com
PPT Indicators for AcidBase Titrations (Sec. 96) PowerPoint Presentation ID4250069 Titration Analysis Function This is what is known as titration. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. Titration is a way of. Titration Analysis Function.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Analysis Function A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. Titration is. Titration Analysis Function.
From www.slideshare.net
Intro to titrations Titration Analysis Function A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. This is what is known as titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something,. Titration Analysis Function.
From chem.libretexts.org
5.7 Quantitative Analysis Using Titration Chemistry LibreTexts Titration Analysis Function Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use it to find the concentration of a. Dissolving the analyte and. Titration Analysis Function.
From www.mometrix.com
Titration Chemistry Review (Video) Titration Analysis Function A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. This is what is known as titration. A “classical” redox titration is one in which a. Titration Analysis Function.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Analysis Function Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. For example, you can use it to find the concentration of a. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. This is what is known as titration. A quantitative chemical analysis in which. Titration Analysis Function.
From www.slideserve.com
PPT Volumetric ANALYSIS/TITRATION PowerPoint Presentation, free download ID3199831 Titration Analysis Function A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. For example, you can use it to find the concentration of a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. A “classical” redox titration. Titration Analysis Function.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Analysis Function Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A “classical” redox titration is one in which a titrant of a known concentration is prepared and. Titration Analysis Function.
From www.pharmaspecialists.com
Potentiometric Titration in Pharmaceutical Analysis Titration Analysis Function For example, you can use it to find the concentration of a. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively. Titration Analysis Function.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Titration Analysis Function Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample.. Titration Analysis Function.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Analysis Function A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. A “classical” redox titration is one in which a titrant of a known concentration is prepared. Titration Analysis Function.
From www.sliderbase.com
Titration Titration Analysis Function This is what is known as titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being analyzed. A “classical” redox titration is one in which a titrant. Titration Analysis Function.
From bramblechemistry.weebly.com
4C6 Titration Titration Analysis Function Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For example, you can use it to find the concentration of a. A quantitative chemical analysis in which a defined. Titration Analysis Function.
From www.calpaclab.com
The Science of Titration Analysis CP Lab Safety Titration Analysis Function Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. This is what is known as titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way. Titration Analysis Function.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID3254207 Titration Analysis Function Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. Titration, process of chemical analysis in which the quantity of some constituent of. Titration Analysis Function.
From chem.libretexts.org
9.4.2 AcidBase Titrations Chemistry LibreTexts Titration Analysis Function Dissolving the analyte and making it react with another species in solution (titrant) of known concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A. Titration Analysis Function.
From www.researchgate.net
Setup of the automated titration (a) before titration, (b) after the... Download Scientific Titration Analysis Function Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the measured sample. A “classical” redox titration is one in which a titrant of a known concentration is prepared and standardized versus some appropriate primary standard. Titration is a way of measuring the concentration of something, usually the. Titration Analysis Function.