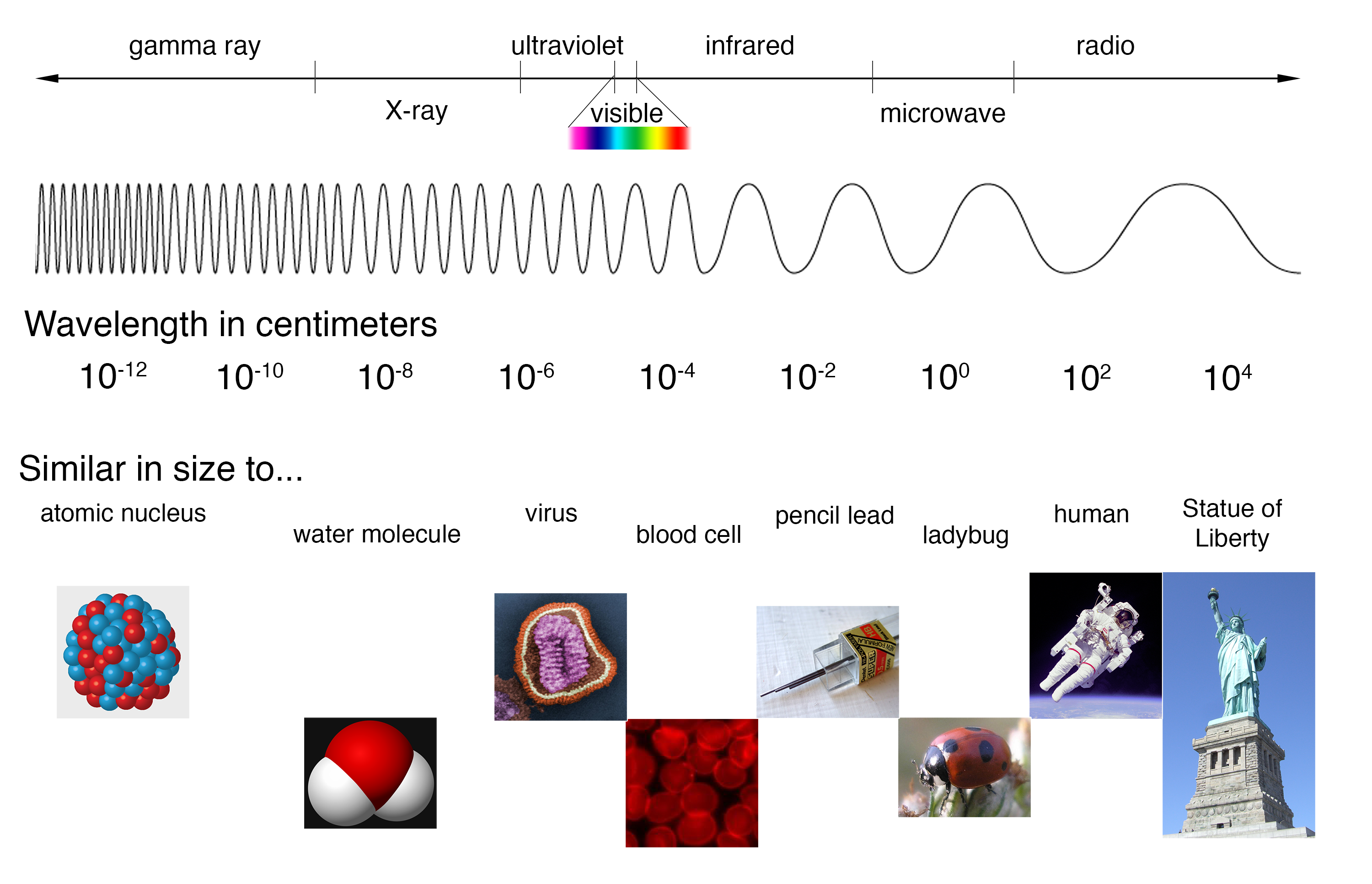
Emission Spectrum Questions . Evidence from atomic line spectra supports the bohr. Electrons move rapidly around the nucleus in energy shells. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. The figure below shows the. If their energy is increased, then they can jump to a higher. All elements either emit or absorb certain frequencies of light. Electrons move rapidly around the nucleus in energy shells. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. What is an emission spectrum? (b) the spectrum of light absorbed by an object. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. When hydrogen gas is placed. (a) the spectrum of light emitted by a hot object. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Heat or electricity can be used to excite an electron to a higher.
from imagine.gsfc.nasa.gov
An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. (a) the spectrum of light emitted by a hot object. All elements either emit or absorb certain frequencies of light. (b) the spectrum of light absorbed by an object. Electrons move rapidly around the nucleus in energy shells. What is an emission spectrum? The figure below shows the. If their energy is increased, then they can jump to a higher. When hydrogen gas is placed.
Spectra Introduction
Emission Spectrum Questions (b) the spectrum of light absorbed by an object. When hydrogen gas is placed. Heat or electricity can be used to excite an electron to a higher. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Electrons move rapidly around the nucleus in energy shells. What is an emission spectrum? All elements either emit or absorb certain frequencies of light. Evidence from atomic line spectra supports the bohr. The figure below shows the. If their energy is increased, then they can jump to a higher. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Electrons move rapidly around the nucleus in energy shells. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. (b) the spectrum of light absorbed by an object. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. (a) the spectrum of light emitted by a hot object.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Emission Spectrum Questions This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Electrons move rapidly around the nucleus in energy shells. When hydrogen gas is placed. If their energy is increased, then they can jump to a higher. The figure below shows the. Evidence from atomic line spectra supports the bohr. What is. Emission Spectrum Questions.
From sekaangry.weebly.com
Atomic emission spectrum chemistry definition sekaangry Emission Spectrum Questions Electrons move rapidly around the nucleus in energy shells. (b) the spectrum of light absorbed by an object. Electrons move rapidly around the nucleus in energy shells. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An atomic emission spectrum is the pattern. Emission Spectrum Questions.
From www.dreamstime.com
The Spectrum Vector Diagram Stock Vector Illustration Emission Spectrum Questions (b) the spectrum of light absorbed by an object. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. The figure below shows the. Electrons. Emission Spectrum Questions.
From mathematica.stackexchange.com
graphics How to plot an emission spectrum? Mathematica Stack Exchange Emission Spectrum Questions An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. (b) the spectrum of light absorbed by an object. Electrons move rapidly around the nucleus in energy shells. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known. Emission Spectrum Questions.
From slideplayer.com
Atomic emission spectrum ppt download Emission Spectrum Questions The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Electrons move rapidly around the nucleus in energy shells. (b) the spectrum of light absorbed. Emission Spectrum Questions.
From edurev.in
Using the Rydberg formula,calculate the wavelengths of first four Emission Spectrum Questions Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. What is an emission spectrum? When hydrogen gas is placed. Electrons move rapidly around the nucleus in energy shells. Electrons move rapidly around the nucleus in energy shells. Evidence from atomic line spectra supports the bohr. An atomic emission spectrum. Emission Spectrum Questions.
From studylib.net
Emission Spectrum Lab and Flame Test Discussion Questions Emission Spectrum Questions An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. (b) the spectrum of light absorbed by. Emission Spectrum Questions.
From socratic.org
What causes the emission of radiant energy that produces Emission Spectrum Questions The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. What is an emission spectrum? (a) the. Emission Spectrum Questions.
From byjus.com
Emission Spectrum Atomic Spectra Comparison Chemistry Byju's Emission Spectrum Questions Electrons move rapidly around the nucleus in energy shells. What is an emission spectrum? Heat or electricity can be used to excite an electron to a higher. (b) the spectrum of light absorbed by an object. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. An atomic emission spectrum is. Emission Spectrum Questions.
From www.reddit.com
Emission Spectra of the Elements in Periodic Table Format r/chemistry Emission Spectrum Questions Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Emission Spectrum Questions.
From ar.inspiredpencil.com
Krypton Spectral Lines Emission Spectrum Questions Heat or electricity can be used to excite an electron to a higher. If their energy is increased, then they can jump to a higher. Evidence from atomic line spectra supports the bohr. The figure below shows the. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The emission spectrum. Emission Spectrum Questions.
From www.nagwa.com
Question Video Identifying Electron Energy Level Transitions of the Emission Spectrum Questions When hydrogen gas is placed. Evidence from atomic line spectra supports the bohr. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. (b) the spectrum of light absorbed by an object. Electrons move rapidly around the nucleus in energy shells. Electrons move rapidly around the nucleus in energy shells. All. Emission Spectrum Questions.
From www.chegg.com
Solved Question 1 The Emission Spectra Shown Below Were O... Emission Spectrum Questions (a) the spectrum of light emitted by a hot object. Heat or electricity can be used to excite an electron to a higher. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Electrons move rapidly around the nucleus in energy shells. An atomic emission spectrum is the pattern of lines. Emission Spectrum Questions.
From sunwindsolar.com
Solar Radiation Spectrum • SunWind Solar Emission Spectrum Questions Electrons move rapidly around the nucleus in energy shells. (b) the spectrum of light absorbed by an object. The figure below shows the. Electrons move rapidly around the nucleus in energy shells. All elements either emit or absorb certain frequencies of light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when. Emission Spectrum Questions.
From imagine.gsfc.nasa.gov
Spectra Introduction Emission Spectrum Questions The figure below shows the. Heat or electricity can be used to excite an electron to a higher. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as. Emission Spectrum Questions.
From brainly.com
An absorption spectrum of oxygen is shown below. What is most likely Emission Spectrum Questions When hydrogen gas is placed. (b) the spectrum of light absorbed by an object. Electrons move rapidly around the nucleus in energy shells. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. Heat or electricity can be used to excite an electron to a higher. This spectrum of radiation. Emission Spectrum Questions.
From es.scribd.com
Atomic Spectra Worksheet Answer Key 0506 PDF Emission Spectrum Questions When hydrogen gas is placed. Heat or electricity can be used to excite an electron to a higher. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. If their energy is increased, then they can jump to a higher. Line spectra is. Emission Spectrum Questions.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectrum Questions Heat or electricity can be used to excite an electron to a higher. Evidence from atomic line spectra supports the bohr. Electrons move rapidly around the nucleus in energy shells. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. This spectrum of radiation. Emission Spectrum Questions.
From www.reddit.com
Periodic table of each elements emission spectrum with regular periodic Emission Spectrum Questions What is an emission spectrum? The figure below shows the. Heat or electricity can be used to excite an electron to a higher. (b) the spectrum of light absorbed by an object. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. All elements either emit or absorb certain frequencies of. Emission Spectrum Questions.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Emission Spectrum Questions What is an emission spectrum? Electrons move rapidly around the nucleus in energy shells. All elements either emit or absorb certain frequencies of light. (b) the spectrum of light absorbed by an object. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. Electrons move rapidly around the nucleus in. Emission Spectrum Questions.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectrum Questions The figure below shows the. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. Heat or electricity can be used to excite an electron to a higher. When hydrogen gas is placed. All elements either emit or absorb certain frequencies of light. Electrons move rapidly around the nucleus in. Emission Spectrum Questions.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Emission Spectrum Questions The figure below shows the. Electrons move rapidly around the nucleus in energy shells. This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains.. Emission Spectrum Questions.
From www.nagwa.com
Question Video Understanding Redshift in Spectral Lines Nagwa Emission Spectrum Questions Electrons move rapidly around the nucleus in energy shells. What is an emission spectrum? Electrons move rapidly around the nucleus in energy shells. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. The figure below shows the. An atomic emission spectrum is the pattern of lines formed when light. Emission Spectrum Questions.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Emission Spectrum Questions (b) the spectrum of light absorbed by an object. Evidence from atomic line spectra supports the bohr. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Electrons move rapidly around the nucleus in energy shells. Line spectra is a phenomenon which occurs. Emission Spectrum Questions.
From www.nagwa.com
Question Video Identifying the Emission Spectrum Corresponding to an Emission Spectrum Questions This spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. Heat or electricity can be used to excite an electron to a higher. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. (b) the spectrum of light absorbed by an. Emission Spectrum Questions.
From www.chegg.com
Solved 1. Consider the following two absorption spectra. Emission Spectrum Questions (a) the spectrum of light emitted by a hot object. Evidence from atomic line spectra supports the bohr. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The figure below shows the. Electrons move rapidly around the nucleus in energy shells. If. Emission Spectrum Questions.
From www.youtube.com
Emission spectrum of hydrogen Chemistry Khan Academy YouTube Emission Spectrum Questions If their energy is increased, then they can jump to a higher. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. (b) the spectrum of light absorbed by an object. Electrons move rapidly around the nucleus in energy shells. This spectrum of radiation emitted by electrons in the excited. Emission Spectrum Questions.
From brainly.com
What is an emission spectrum? Use the Bohr model to explain why the Emission Spectrum Questions Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. Electrons move rapidly around the nucleus in energy shells. (a) the spectrum of. Emission Spectrum Questions.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Emission Spectrum Questions Evidence from atomic line spectra supports the bohr. When hydrogen gas is placed. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. (b) the spectrum of light absorbed by an object. Electrons move rapidly around the nucleus in energy shells. (a) the spectrum of light emitted by a hot. Emission Spectrum Questions.
From mrsmorrittscience.weebly.com
Emission Spectra of the Elements Arranged on the Periodic Table Mrs Emission Spectrum Questions The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. All elements either emit or absorb certain frequencies of light. What is an emission spectrum? (b) the spectrum of light absorbed by an object. (a) the spectrum of light emitted by a hot object.. Emission Spectrum Questions.
From study.com
Quiz & Worksheet Atomic Spectra Characteristics & Types Emission Spectrum Questions All elements either emit or absorb certain frequencies of light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. (b) the spectrum of light absorbed by an object. An atomic emission spectrum is the pattern of lines formed when light passes through a. Emission Spectrum Questions.
From www.chegg.com
Solved Atomic Emission Spectra Questions and Problems 1. Emission Spectrum Questions When hydrogen gas is placed. What is an emission spectrum? Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different colours. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. An atomic emission spectrum. Emission Spectrum Questions.
From www.resonancescience.org
What is Resonance and Why is it so Important? Emission Spectrum Questions (b) the spectrum of light absorbed by an object. All elements either emit or absorb certain frequencies of light. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. If their energy is increased, then they can jump to a higher. When hydrogen gas. Emission Spectrum Questions.
From www.chegg.com
Solved Please help me solve for Sodium, Mercury and Neon! Emission Spectrum Questions (b) the spectrum of light absorbed by an object. An atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it contains. The figure below shows the. Line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond to different. Emission Spectrum Questions.
From www.nagwa.com
Question Video Identifying a Spectrum as an Emission or Absorption Emission Spectrum Questions The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or electricity. Electrons move rapidly around the nucleus in energy shells. Evidence from atomic line spectra supports the bohr. When hydrogen gas is placed. Electrons move rapidly around the nucleus in energy shells. Heat or electricity. Emission Spectrum Questions.