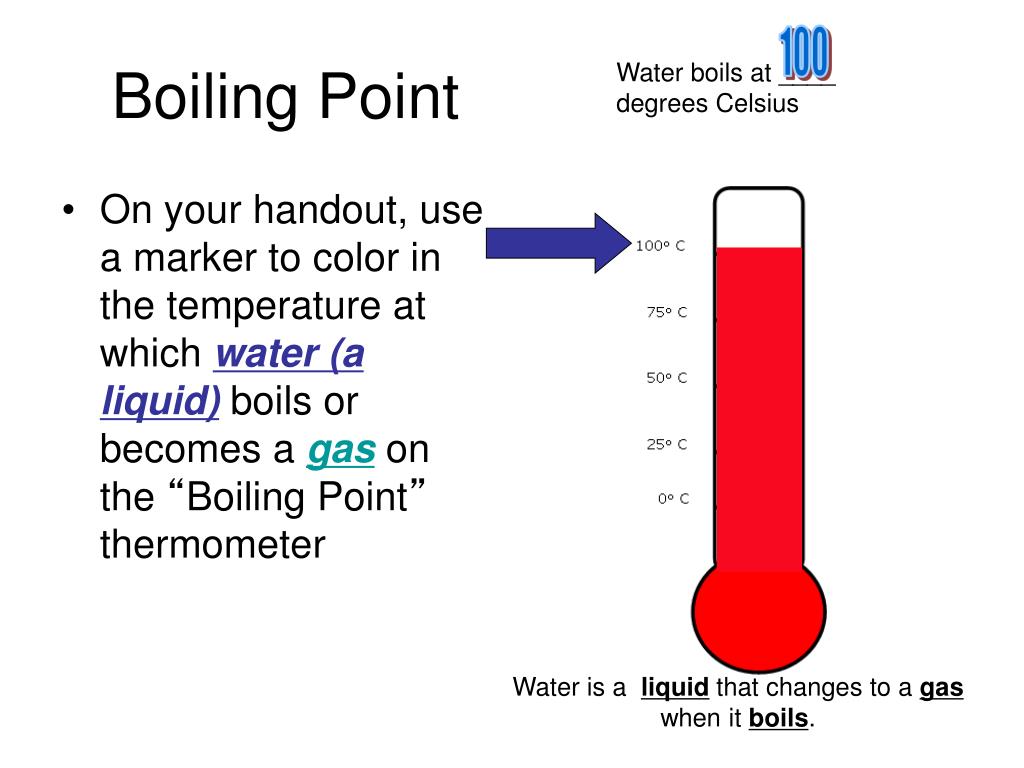
What Is Boiling Point Definition . The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is the temperature for a particular liquid to boil at. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. As the altitude increases, the boiling. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The change from a liquid phase to a gaseous phase. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure.
from www.slideserve.com
Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Normally when we boil a liquid, we do so at atmospheric pressure. As the altitude increases, the boiling. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The change from a liquid phase to a gaseous phase. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. The boiling point is the temperature for a particular liquid to boil at.
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free
What Is Boiling Point Definition Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is the temperature for a particular liquid to boil at. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. As the altitude increases, the boiling. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The change from a liquid phase to a gaseous phase. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is.
From www.animalia-life.club
Boiling Point Of Water For Kids What Is Boiling Point Definition If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. As the altitude increases, the boiling. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The boiling point is the temperature at which the vapor pressure. What Is Boiling Point Definition.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is Boiling Point Definition Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. As the altitude increases, the boiling. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The boiling point is the. What Is Boiling Point Definition.
From www.thoughtco.com
Boiling Definition in Chemistry What Is Boiling Point Definition Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is the temperature for a particular liquid to boil at. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. If this pressure is the standard pressure of 1 atm (101.3 kpa), then. What Is Boiling Point Definition.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) What Is Boiling Point Definition As the altitude increases, the boiling. The change from a liquid phase to a gaseous phase. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature. What Is Boiling Point Definition.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point What Is Boiling Point Definition For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. As the altitude increases, the boiling. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled. What Is Boiling Point Definition.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point What Is Boiling Point Definition For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. As the altitude increases, the boiling. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled. What Is Boiling Point Definition.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Is Boiling Point Definition The change from a liquid phase to a gaseous phase. The boiling point is the temperature for a particular liquid to boil at. As the altitude increases, the boiling. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling point is the temperature at which the vapor pressure. What Is Boiling Point Definition.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is Boiling Point Definition As the altitude increases, the boiling. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The change from a liquid phase to a gaseous phase. Normally when we boil a liquid,. What Is Boiling Point Definition.
From www.youtube.com
Boiling Point of Organic Compounds YouTube What Is Boiling Point Definition Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. As the altitude increases, the boiling. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature. What Is Boiling Point Definition.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is Boiling Point Definition As the altitude increases, the boiling. The boiling point is the temperature for a particular liquid to boil at. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point.. What Is Boiling Point Definition.
From www.slideserve.com
PPT Properties and Changes in Matter PowerPoint Presentation, free What Is Boiling Point Definition Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. As the altitude increases, the boiling. Normally when we boil a liquid, we do so at atmospheric pressure. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. Boiling is the. What Is Boiling Point Definition.
From www.youtube.com
Boiling and Condensation Difference between Boiling and Condensation What Is Boiling Point Definition For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1. What Is Boiling Point Definition.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin What Is Boiling Point Definition The change from a liquid phase to a gaseous phase. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. For example, the boiling point for water,. What Is Boiling Point Definition.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Boiling Point Definition The change from a liquid phase to a gaseous phase. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. Boiling is the process by which a liquid turns into a vapor when it is heated. What Is Boiling Point Definition.
From study.com
Boiling Point Elevation Definition, Formula & Equation Lesson What Is Boiling Point Definition If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The change from a liquid phase to a gaseous phase. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The boiling point is the temperature for. What Is Boiling Point Definition.
From www.slideserve.com
PPT Periodic Table Trends Melting & Boiling Point PowerPoint What Is Boiling Point Definition For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the.. What Is Boiling Point Definition.
From www.pinterest.com
Boiling Point Boiling point, Distillation, Water boiling What Is Boiling Point Definition The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point, temperature at which the pressure. What Is Boiling Point Definition.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is Boiling Point Definition The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. Boiling. What Is Boiling Point Definition.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Is Boiling Point Definition The change from a liquid phase to a gaseous phase. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. For example, the boiling point for water,. What Is Boiling Point Definition.
From www.yiannislucacos.gr
How to boil Boiling as a basic cooking method Yiannis Lucacos What Is Boiling Point Definition The change from a liquid phase to a gaseous phase. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Normally when we boil a liquid, we do so at atmospheric pressure. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius.. What Is Boiling Point Definition.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is Boiling Point Definition The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point, temperature at which the. What Is Boiling Point Definition.
From matmake.com
Boiling Point Definition, Measurements, and Applications What Is Boiling Point Definition The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted. What Is Boiling Point Definition.
From chemistnotes.com
Boiling Point Definition, Boiling point as physical properties What Is Boiling Point Definition As the altitude increases, the boiling. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by. What Is Boiling Point Definition.
From chemhero.blogspot.com
Definition Of Boiling In Chemistry Chem Hero What Is Boiling Point Definition Normally when we boil a liquid, we do so at atmospheric pressure. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The change from a liquid phase to a gaseous phase. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the. What Is Boiling Point Definition.
From ppt-online.org
theory of ideal gases презентация онлайн What Is Boiling Point Definition The boiling point is the temperature for a particular liquid to boil at. As the altitude increases, the boiling. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding. What Is Boiling Point Definition.
From www.worksheetsplanet.com
What is Boiling Point What Is Boiling Point Definition If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The boiling. What Is Boiling Point Definition.
From www.differencebetween.com
Difference Between Boiling Point and Melting Point Compare the What Is Boiling Point Definition Normally when we boil a liquid, we do so at atmospheric pressure. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The change from a liquid phase to a gaseous phase. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is.. What Is Boiling Point Definition.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID What Is Boiling Point Definition As the altitude increases, the boiling. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. The change from a liquid phase to a gaseous phase. Boiling is the process by which. What Is Boiling Point Definition.
From gamesmartz.com
Normal Boiling Point Definition & Image GameSmartz What Is Boiling Point Definition Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. The. What Is Boiling Point Definition.
From gamesmartz.com
Boiling Point Elevation Definition & Image GameSmartz What Is Boiling Point Definition As the altitude increases, the boiling. The change from a liquid phase to a gaseous phase. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point is the temperature for a particular liquid to boil. What Is Boiling Point Definition.
From www.healthbenefitstimes.com
Boiling Point Definition of Boiling Point What Is Boiling Point Definition The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. The change from a liquid phase to a gaseous phase. As the altitude increases, the boiling. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. Boiling is the process by which. What Is Boiling Point Definition.
From www.animalia-life.club
Boiling Point Of Water Examples What Is Boiling Point Definition For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. As the altitude increases, the boiling. Boiling is the process by which a liquid turns into a vapor when it is heated to its boiling point. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by. What Is Boiling Point Definition.
From www.sliderbase.com
Colligative Properties of Solutions Presentation Chemistry What Is Boiling Point Definition The change from a liquid phase to a gaseous phase. The boiling point is the temperature at which the vapor pressure of a liquid equals the external pressure surrounding the liquid. As the altitude increases, the boiling. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the. If. What Is Boiling Point Definition.
From www.youtube.com
Boiling point — BOILING POINT definition YouTube What Is Boiling Point Definition The change from a liquid phase to a gaseous phase. The boiling point is the temperature for a particular liquid to boil at. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The boiling point is the temperature at which the vapor pressure of a liquid equals the. What Is Boiling Point Definition.
From byjus.com
Determination Of Boiling Point Of An Organic Compound Chemistry What Is Boiling Point Definition The change from a liquid phase to a gaseous phase. The boiling point is the temperature at which the vapor pressure of a liquid is equal to the external pressure. Normally when we boil a liquid, we do so at atmospheric pressure. For example, the boiling point for water, at a pressure of 1 atm, is 100 degrees celsius. If. What Is Boiling Point Definition.