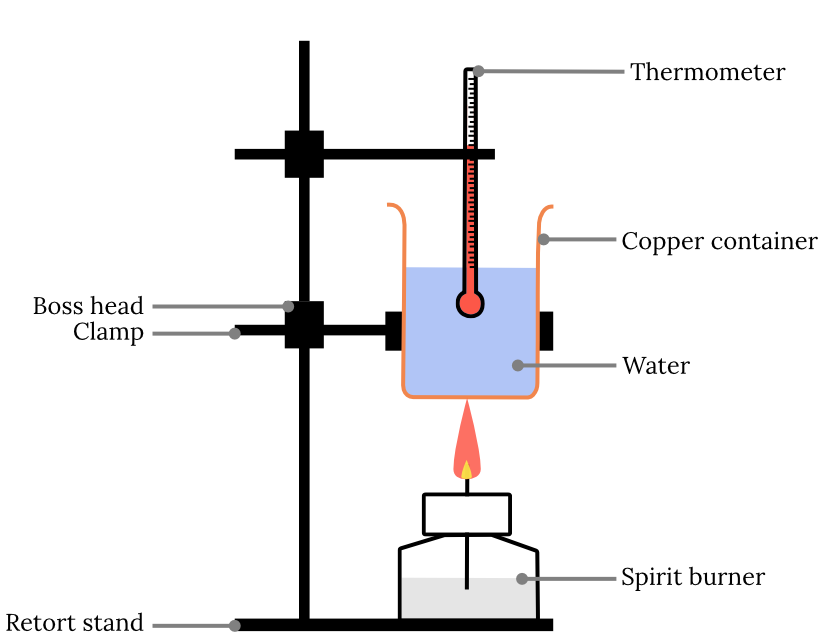
Calorimeter Used To Determine . Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimeters play a crucial role in understanding the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). To do so, the heat is exchanged with a calibrated object (calorimeter).
from www.learnable.education
Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimeters play a crucial role in understanding the. Calorimetry is used to measure amounts of heat transferred to or from a substance. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged with a calibrated object (calorimeter).
Year 11 Chemistry Practical Investigation Calorimetry Experiment
Calorimeter Used To Determine Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters play a crucial role in understanding the. Calorimetry is used to measure amounts of heat transferred to or from a substance. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged with a calibrated object (calorimeter).
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Used To Determine To do so, the heat is exchanged with a calibrated object (calorimeter). This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters are used. Calorimeter Used To Determine.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume Calorimeter Used To Determine Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is the set of. Calorimeter Used To Determine.
From www.dreamstime.com
Calorimeter is Measuring that Can Be Used To Determine Heat of Reaction Calorimeter Used To Determine Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters play a crucial role in understanding the. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the set of techniques used to measure enthalpy changes. Calorimeter Used To Determine.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Used To Determine Calorimeters play a crucial role in understanding the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. This value and the. Calorimeter Used To Determine.
From users.highland.edu
Calorimetry Calorimeter Used To Determine One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeters play a crucial role in understanding the. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the set. Calorimeter Used To Determine.
From depositphotos.com
Calorimeter Measuring Can Used Determine Heat Reaction Liquid Solid Calorimeter Used To Determine Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimeters. Calorimeter Used To Determine.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Calorimeter Used To Determine To do so, the heat is exchanged with a calibrated object (calorimeter). This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used. Calorimeter Used To Determine.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimeter Used To Determine Calorimeters play a crucial role in understanding the. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters are used to measure the heat of combustion, heat capacity, and. Calorimeter Used To Determine.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimeter Used To Determine To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters play a crucial role in understanding the. This value and the. Calorimeter Used To Determine.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Used To Determine To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters play a crucial role in understanding the. To do so, the heat is exchanged with a calibrated object (calorimeter). This value and the measured increase in temperature of the calorimeter can be used. Calorimeter Used To Determine.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Calorimeter Used To Determine Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters play a crucial role. Calorimeter Used To Determine.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimeter Used To Determine One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. This value and the measured increase in temperature of the calorimeter can. Calorimeter Used To Determine.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Used To Determine Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeters. Calorimeter Used To Determine.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Used To Determine Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimeters play a crucial role in understanding the. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. Calorimeter Used To Determine.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter Used To Determine Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. Calorimeter Used To Determine.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Calorimeter Used To Determine Calorimetry is used to measure amounts of heat transferred to or from a substance. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeters play a crucial role in understanding the. One technique we can use to measure. Calorimeter Used To Determine.
From chemistrytalk.org
Calorimetry ChemTalk Calorimeter Used To Determine Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimeters play a crucial role in understanding the. One technique we can. Calorimeter Used To Determine.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimeter Used To Determine This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of. Calorimeter Used To Determine.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Calorimeter Used To Determine Calorimeters play a crucial role in understanding the. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. This value and the measured increase in temperature of the calorimeter can. Calorimeter Used To Determine.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Used To Determine One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. To do so, the heat is exchanged. Calorimeter Used To Determine.
From www.scienceabc.com
How Do We Calculate How Many Carbs Are In The Food? Calorimeter Used To Determine Calorimeters play a crucial role in understanding the. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure amounts of. Calorimeter Used To Determine.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Used To Determine Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeters play a crucial role in understanding the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This value and. Calorimeter Used To Determine.
From www.dreamstime.com
Calorimeter is Measuring that Can Be Used To Determine Heat of Reaction Calorimeter Used To Determine This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters play a crucial role in understanding the. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the. Calorimeter Used To Determine.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Calorimeter Used To Determine This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters are used to measure the heat of combustion, heat capacity, and heats. Calorimeter Used To Determine.
From courses.lumenlearning.com
Calorimetry Chemistry I Calorimeter Used To Determine Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical. Calorimeter Used To Determine.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Calorimeter Used To Determine One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is used to measure amounts of heat transferred to or from. Calorimeter Used To Determine.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter Used To Determine One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is used to measure. Calorimeter Used To Determine.
From www.alamy.com
Rumford's calorimeter, used to determine the amount of heat produced by Calorimeter Used To Determine Calorimetry is used to measure amounts of heat transferred to or from a substance. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. To do so, the heat is exchanged with a calibrated object (calorimeter).. Calorimeter Used To Determine.
From www.alamy.com
Mahler bomb calorimeter, a device used to determine the high calorific Calorimeter Used To Determine To do so, the heat is exchanged with a calibrated object (calorimeter). This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. Calorimeters play a crucial role in understanding the. Calorimetry is the set of techniques. Calorimeter Used To Determine.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Used To Determine To do so, the heat is exchanged with a calibrated object (calorimeter). This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction.. Calorimeter Used To Determine.
From saylordotorg.github.io
Calorimetry Calorimeter Used To Determine One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is the set of techniques used to measure enthalpy changes during. Calorimeter Used To Determine.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Used To Determine Calorimeters are used to measure the heat of combustion, heat capacity, and heats of reaction. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a. Calorimeter Used To Determine.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimeter Used To Determine Calorimeters play a crucial role in understanding the. Calorimetry is used to measure amounts of heat transferred to or from a substance. This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use. Calorimeter Used To Determine.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Used To Determine This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a calibrated object (calorimeter). To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeters play a. Calorimeter Used To Determine.
From studyfinder.org
How to Use a Calorimetry Gizmo to Get the Answers You Need Calorimeter Used To Determine To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimeters play a crucial role in understanding the. To do so, the heat is exchanged with a calibrated object (calorimeter). This value and the measured increase in temperature of the calorimeter can be used in equation \(\ref{5.5.9}\) to determine. Calorimetry is used to measure amounts of heat transferred. Calorimeter Used To Determine.