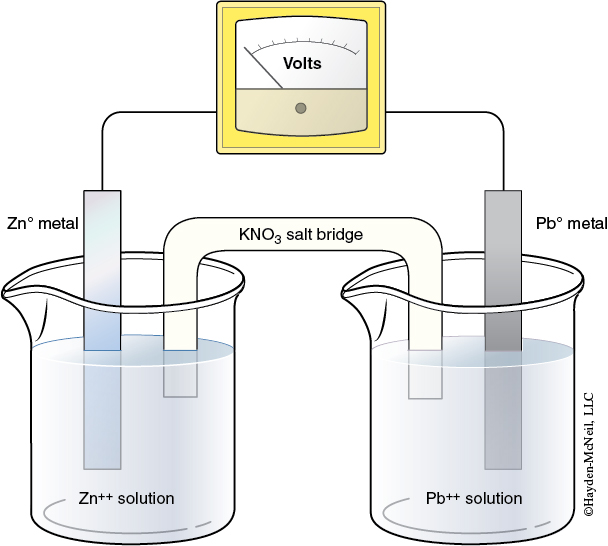
Voltaic Cell Real Life Example . This type of electrochemical cell is often called a voltaic cell after. The voltaic cell (see figure above) consists of two separate compartments. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A voltaic cell is an example of what is known as an electrochemical cell. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy.
from www.chegg.com
A voltaic cell is an example of what is known as an electrochemical cell. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell produces electricity as a redox reaction occurs. This type of electrochemical cell is often called a voltaic cell after. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions.
The above image is an example of a Voltaic Cell.
Voltaic Cell Real Life Example It gets this classification owing to the fact that, within it, a chemical reaction takes place which. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. A voltaic cell produces electricity as a redox reaction occurs. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell is an example of what is known as an electrochemical cell. This type of electrochemical cell is often called a voltaic cell after.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Voltaic Cell Real Life Example This type of electrochemical cell is often called a voltaic cell after. A voltaic cell produces electricity as a redox reaction occurs. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell is an example of what is known as an electrochemical cell. A voltaic cell (also known as a galvanic cell) is an electrochemical cell. Voltaic Cell Real Life Example.
From www.slideserve.com
PPT Voltaic Cells PowerPoint Presentation, free download ID5976249 Voltaic Cell Real Life Example A voltaic cell produces electricity as a redox reaction occurs. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell is an example of what is known as an electrochemical cell. A voltaic cell (also known as. Voltaic Cell Real Life Example.
From galvinconanstuart.blogspot.com
Label The Diagram According To The Components And Processes Of A Voltaic Cell Real Life Example This type of electrochemical cell is often called a voltaic cell after. A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The voltaic cell (see figure above) consists of two separate compartments. The voltage of a voltaic cell. Voltaic Cell Real Life Example.
From www.shutterstock.com
1,233 Galvanic Cells Images, Stock Photos & Vectors Shutterstock Voltaic Cell Real Life Example The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell produces electricity as a redox reaction occurs. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. It gets this classification owing to the fact that, within it, a chemical reaction. Voltaic Cell Real Life Example.
From drcalef.com
A Voltaic Cell Voltaic Cell Real Life Example A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The voltaic cell (see figure above) consists of two separate compartments. This type of electrochemical cell is often called a voltaic cell after. It gets this classification owing to. Voltaic Cell Real Life Example.
From wisc.pb.unizin.org
Day 39 Voltaic Cells, HalfCell Potentials Chemistry 109, Fall 2020 Voltaic Cell Real Life Example It gets this classification owing to the fact that, within it, a chemical reaction takes place which. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The voltage of a voltaic cell can be determined by the. Voltaic Cell Real Life Example.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download Voltaic Cell Real Life Example This type of electrochemical cell is often called a voltaic cell after. A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A voltaic cell is an example of what is known as an electrochemical cell. The voltaic cell (see figure above) consists. Voltaic Cell Real Life Example.
From www.chegg.com
The above image is an example of a Voltaic Cell. Voltaic Cell Real Life Example A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A voltaic cell is an example of what is known as an electrochemical cell. The voltaic cell (see figure above) consists of two separate compartments. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate. Voltaic Cell Real Life Example.
From overallscience.com
Voltaic Cell, Its Construction and Defects Overall Science Voltaic Cell Real Life Example A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell is an example of what is. Voltaic Cell Real Life Example.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Voltaic Cell Real Life Example A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The voltaic cell (see figure above) consists of two separate compartments. This type of electrochemical cell is often called a voltaic cell after. A voltaic cell is an example of what is known as an electrochemical cell. A voltaic cell is. Voltaic Cell Real Life Example.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Voltaic Cell Real Life Example A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell is an example of what is known as an electrochemical cell. A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy.. Voltaic Cell Real Life Example.
From chem.libretexts.org
20.3 Voltaic Cells Chemistry LibreTexts Voltaic Cell Real Life Example This type of electrochemical cell is often called a voltaic cell after. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. The voltage of. Voltaic Cell Real Life Example.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Voltaic Cell Real Life Example A voltaic cell is an example of what is known as an electrochemical cell. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A voltaic cell produces electricity as a redox. Voltaic Cell Real Life Example.
From wisc.pb.unizin.org
Day 39 Voltaic Cells Chemistry 109 Voltaic Cell Real Life Example A voltaic cell is an example of what is known as an electrochemical cell. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell (also known as a galvanic cell) is an electrochemical. Voltaic Cell Real Life Example.
From www.slideserve.com
PPT Introductory Chemistry , 3 rd Edition Nivaldo Tro PowerPoint Voltaic Cell Real Life Example A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. This type of electrochemical cell is often called a voltaic cell after.. Voltaic Cell Real Life Example.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Voltaic Cell Real Life Example This type of electrochemical cell is often called a voltaic cell after. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell produces electricity as a redox reaction occurs. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The voltaic. Voltaic Cell Real Life Example.
From www.carolina.com
Voltaic Cell Set, Student Voltaic Cell Real Life Example A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. A voltaic cell is an example of what is known as an electrochemical cell. This type of electrochemical cell is often called a voltaic. Voltaic Cell Real Life Example.
From fphoto.photoshelter.com
science chemistry redox reaction electrochemical cell Fundamental Voltaic Cell Real Life Example This type of electrochemical cell is often called a voltaic cell after. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell is an example of what is known as an electrochemical cell. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to. Voltaic Cell Real Life Example.
From www.ck12.org
Voltaic or Galvanic Cells Example 1 ( Video ) Chemistry CK12 Voltaic Cell Real Life Example A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell is an example of what is known as an electrochemical cell. A voltaic cell (also known as a galvanic. Voltaic Cell Real Life Example.
From electricala2z.com
Voltaic Cell Construction Working Examples Voltaic Cell Real Life Example A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. This type of electrochemical cell is often called a voltaic cell after. The voltaic cell (see figure above) consists of two separate compartments. The. Voltaic Cell Real Life Example.
From byjus.com
The electrolyte of simple voltaic cell is Voltaic Cell Real Life Example A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. This type of electrochemical cell is often called a voltaic cell after. A voltaic cell is an example of what is known as an electrochemical cell. The voltaic cell (see figure above) consists of two separate compartments. It gets this classification. Voltaic Cell Real Life Example.
From www.youtube.com
Ch. 203 Inert Electrodes/Voltaic Cells YouTube Voltaic Cell Real Life Example A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. It gets this classification owing to the fact that,. Voltaic Cell Real Life Example.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Voltaic Cell Real Life Example The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell is an example of what is known as an electrochemical. Voltaic Cell Real Life Example.
From generic.wordpress.soton.ac.uk
Electrochemistry explanations, videos and everyday life examples Voltaic Cell Real Life Example A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. This type of electrochemical cell is often called a voltaic cell after. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. A voltaic cell produces electricity as a redox reaction occurs.. Voltaic Cell Real Life Example.
From www.youtube.com
Voltaic Cell Example YouTube Voltaic Cell Real Life Example A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. The voltaic cell. Voltaic Cell Real Life Example.
From 2012books.lardbucket.org
Applications of Redox Reactions Voltaic Cells Voltaic Cell Real Life Example This type of electrochemical cell is often called a voltaic cell after. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to. Voltaic Cell Real Life Example.
From www.chemistrylearner.com
Galvanic Cell (Voltaic Cell) Chemistry Learner Voltaic Cell Real Life Example A voltaic cell is an example of what is known as an electrochemical cell. The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate. Voltaic Cell Real Life Example.
From www.youtube.com
12 03 Voltaic Cells YouTube Voltaic Cell Real Life Example The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell is an example of what is known as an electrochemical cell. The voltaic cell (see figure above) consists of two separate compartments. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. A. Voltaic Cell Real Life Example.
From www.youtube.com
Part 1 of 2 Galvanic / Voltaic Cells Examples YouTube Voltaic Cell Real Life Example The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. This type of. Voltaic Cell Real Life Example.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Voltaic Cell Real Life Example The voltaic cell (see figure above) consists of two separate compartments. A voltaic cell produces electricity as a redox reaction occurs. The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell. Voltaic Cell Real Life Example.
From www.youtube.com
Uses of Electrochemistry in our daily life (Electrochemistry part 2 for Voltaic Cell Real Life Example It gets this classification owing to the fact that, within it, a chemical reaction takes place which. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A voltaic cell is an example of what is known as an electrochemical cell. A voltaic cell produces electricity as a redox. Voltaic Cell Real Life Example.
From diagramtabormanages.z21.web.core.windows.net
Diagram Of Voltaic Cell Voltaic Cell Real Life Example This type of electrochemical cell is often called a voltaic cell after. A voltaic cell (also known as a galvanic cell) is an electrochemical cell that uses spontaneous redox reactions to generate electricity. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. A voltaic cell is an example of what. Voltaic Cell Real Life Example.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Voltaic Cell Real Life Example The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A voltaic cell is an example of what is known as an electrochemical cell. A voltaic cell produces electricity as a redox reaction occurs. This type. Voltaic Cell Real Life Example.
From studylib.net
Voltaic Cell Part 2 Voltaic Cell Real Life Example A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. It gets this classification owing to the fact that, within it, a chemical reaction takes place which. A voltaic cell is an example of what is known as an electrochemical cell. A galvanic (voltaic) cell uses the energy released during a spontaneous redox. Voltaic Cell Real Life Example.
From www.youtube.com
Working of Simple Voltaic Cell YouTube Voltaic Cell Real Life Example A voltaic cell produces electricity as a redox reaction occurs. A voltaic cell is an electrochemical cell that uses a spontaneous redox reaction to produce electrical energy. A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (\(δg < 0\)) to generate electricity. The voltage of a voltaic cell can be determined by the reduction potentials of. Voltaic Cell Real Life Example.