Zinc Chloride Hydrate Formula . Data from nist standard reference database 69: Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zinc chlorides, of which at nine crystalline forms are. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Hydrates generally contain water in stoichiometric amounts;
from lab.honeywell.com
The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Data from nist standard reference database 69: Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Hydrates generally contain water in stoichiometric amounts; Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: 1) determine the percentage of water in cobalt(ii) chloride dihydrate: The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Zinc chlorides, of which at nine crystalline forms are.
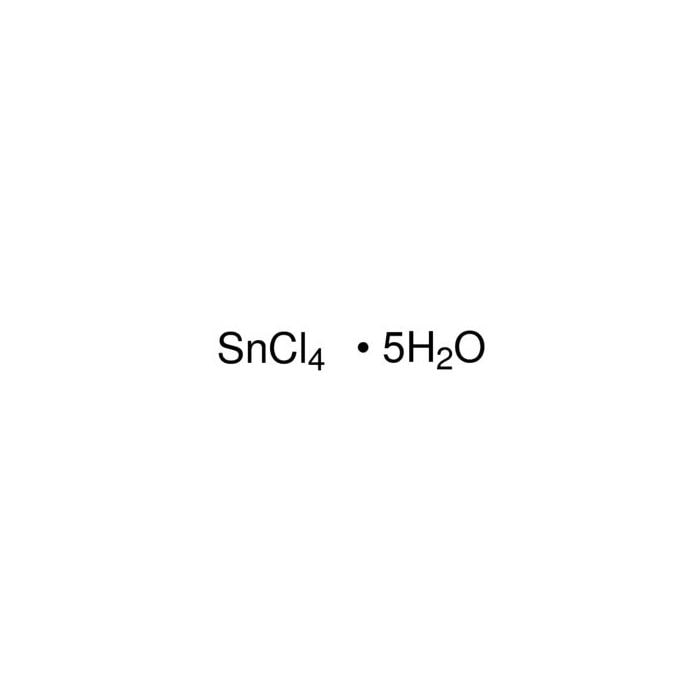
Tin(IV) chloride5hydrate 14550 Honeywell Research Chemicals
Zinc Chloride Hydrate Formula Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Hydrates generally contain water in stoichiometric amounts; Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Data from nist standard reference database 69: The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Zinc chlorides, of which at nine crystalline forms are.
From slideplayer.com
HYDRATES Unit 7 Lesson ppt download Zinc Chloride Hydrate Formula The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Hydrates generally contain water in. Zinc Chloride Hydrate Formula.
From www.biosynth.com
GAA08756 6087565 4DiazoN,Ndimethylaniline Chloride Zinc Zinc Chloride Hydrate Formula Data from nist standard reference database 69: The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Zinc chlorides, of which at nine crystalline forms are. Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. 1) determine the. Zinc Chloride Hydrate Formula.
From www.glpbio.com
Gold(III) chloride trihydrate CAS NO.16961254 GlpBio Zinc Chloride Hydrate Formula Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Hydrates generally contain water in stoichiometric amounts; The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be. Zinc Chloride Hydrate Formula.
From www.slideserve.com
PPT The Formula for a Hydrate PowerPoint Presentation, free download Zinc Chloride Hydrate Formula Zinc chlorides, of which at nine crystalline forms are. The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Data from nist standard reference database 69: Solid zncl 2. Zinc Chloride Hydrate Formula.
From www.sigmaaldrich.co.th
ZINC CHLORIDE, 98+ Merck Life Sciences Thailand Zinc Chloride Hydrate Formula Nist chemistry webbook the national institute of standards and technology (nist) uses its best. The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n. Zinc Chloride Hydrate Formula.
From www.scielo.br
SciELO Brasil Synthesis and Characterization of Zinc Oxide Obtained Zinc Chloride Hydrate Formula The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Hydrates generally contain water in stoichiometric amounts; 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Data from nist standard reference database 69: Zinc chlorides, of which at nine crystalline forms are. Solid zncl 2. Zinc Chloride Hydrate Formula.
From www.coursehero.com
[Solved] Write the chemical formula for each hydrate. Use the proper Zinc Chloride Hydrate Formula Hydrates generally contain water in stoichiometric amounts; Zinc chlorides, of which at nine crystalline forms are. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Data from nist standard reference database 69: 1) determine the percentage of water in cobalt(ii) chloride dihydrate: The five known hydrates of zinc chloride have the general formula zncl. Zinc Chloride Hydrate Formula.
From www.laboratoriumdiscounter.nl
Buy Zinc Chloride CAS 7646857 ? Pure zinc chloride CAS 7646857 in Zinc Chloride Hydrate Formula Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Data from nist standard reference database 69: Zinc chlorides, of which at nine crystalline forms are. Hydrates generally contain water in stoichiometric amounts; The five known hydrates of. Zinc Chloride Hydrate Formula.
From www.slideserve.com
PPT Formula of a Hydrate PowerPoint Presentation, free download ID Zinc Chloride Hydrate Formula The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zinc chlorides, of which at nine. Zinc Chloride Hydrate Formula.
From www.funcmater.com
buy Zinc chloride hydrate Crystalline manufacturers FUNCMATER Zinc Chloride Hydrate Formula Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zinc chloride is the name of chemical compound with the formula zn cl 2 and. Zinc Chloride Hydrate Formula.
From examquiz.netlify.app
Determining the formula of a hydrate examquiz Zinc Chloride Hydrate Formula Data from nist standard reference database 69: The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Zinc chlorides, of which at nine crystalline forms are. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Nist chemistry webbook the national. Zinc Chloride Hydrate Formula.
From www.youtube.com
Empirical Formula Experiment copper chloride hydrate YouTube Zinc Chloride Hydrate Formula Zinc chlorides, of which at nine crystalline forms are. Data from nist standard reference database 69: 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Hydrates generally contain water in stoichiometric amounts; Nist chemistry webbook the national institute of standards and technology (nist) uses. Zinc Chloride Hydrate Formula.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Chloride Hydrate Formula 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: The five known hydrates. Zinc Chloride Hydrate Formula.
From www.dreamstime.com
Molecular Formula of Zinc Chloride Stock Illustration Illustration of Zinc Chloride Hydrate Formula Data from nist standard reference database 69: The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original. Zinc Chloride Hydrate Formula.
From www.nanochemazone.com
Zinc Chloride Hydrate Powder Price 40 High Purity Nanochemazone Zinc Chloride Hydrate Formula The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Zinc chlorides, of which at nine. Zinc Chloride Hydrate Formula.
From www.shutterstock.com
122 Zinc Chloride Images, Stock Photos & Vectors Shutterstock Zinc Chloride Hydrate Formula Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Hydrates generally contain water in stoichiometric amounts; Nist chemistry webbook the national institute of standards and technology (nist) uses its best. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Zinc chloride is the name of chemical compound with the formula zn cl 2. Zinc Chloride Hydrate Formula.
From www.indiamart.com
Zinc Chloride Pure at Rs 105/kg Zinc Chloride in Navi Mumbai ID Zinc Chloride Hydrate Formula Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Data from nist standard reference database 69: Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Zinc chlorides, of which at nine crystalline forms are. Zinc chloride is the name of chemical compound with the formula zn cl 2 and. Zinc Chloride Hydrate Formula.
From www.fishersci.co.uk
Zinc Chloride solution 0.1mol/L, Fisher Chemical Fisher Scientific Zinc Chloride Hydrate Formula Hydrates generally contain water in stoichiometric amounts; Zinc chlorides, of which at nine crystalline forms are. Data from nist standard reference database 69: Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Nist chemistry webbook the national institute of standards and technology (nist) uses its best. The formula of a hydrate can. Zinc Chloride Hydrate Formula.
From www.carolina.com
Zinc Chloride, Reagent Grade, 500 g Zinc Chloride Hydrate Formula The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Data from nist standard reference database 69: The formula of a hydrate can be determined by dehydrating a known mass. Zinc Chloride Hydrate Formula.
From lab.honeywell.com
Tin(IV) chloride5hydrate 14550 Honeywell Research Chemicals Zinc Chloride Hydrate Formula The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Data from nist standard reference database 69: Zinc chloride is the name of chemical compound. Zinc Chloride Hydrate Formula.
From askfilo.com
zinc chloride(IV). 30. Correct formula of tetraamminechloridonitroplatinu.. Zinc Chloride Hydrate Formula The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Hydrates generally contain water in stoichiometric amounts; The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and. Zinc Chloride Hydrate Formula.
From www.shutterstock.com
Zinc Chloride Zncl2 Structural Chemical Formula Stock Vector (Royalty Zinc Chloride Hydrate Formula Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Data from nist standard reference database 69: 1) determine the percentage of water in cobalt(ii) chloride dihydrate: The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and. Zinc Chloride Hydrate Formula.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Chloride Hydrate Formula Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Hydrates generally contain water in stoichiometric amounts; Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Nist chemistry webbook the national institute of standards and technology (nist) uses its best. The formula of a hydrate can be. Zinc Chloride Hydrate Formula.
From brainly.com
15.00g of hydrated zinc sulfate loses 6.579 H2O during heating what is Zinc Chloride Hydrate Formula Hydrates generally contain water in stoichiometric amounts; Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Zinc chlorides, of which at nine crystalline forms are. Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Data from nist. Zinc Chloride Hydrate Formula.
From jkenterprises.com.pk
Zinc chloride, anhydrous, 98+ J K Enterprises Chemical J K Zinc Chloride Hydrate Formula Zinc chlorides, of which at nine crystalline forms are. The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Hydrates generally contain water in stoichiometric amounts; Data from nist standard reference database 69: Nist chemistry webbook the national institute of standards and technology (nist) uses its. Zinc Chloride Hydrate Formula.
From www.glentham.com
Zinc citrate trihydrate (CAS 546463) Glentham Life Sciences Zinc Chloride Hydrate Formula The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Nist chemistry webbook the national institute of standards and technology (nist) uses its best. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Solid zncl 2 exhibits polymorphism and. Zinc Chloride Hydrate Formula.
From www.sunshinetrading.co
Zinc Chloride Sunshine Trading Company Zinc Chloride Hydrate Formula Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Hydrates generally contain water in stoichiometric amounts; Data from nist standard reference database 69: Zinc chlorides, of which at nine crystalline forms are. Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. The formula of a hydrate can. Zinc Chloride Hydrate Formula.
From camachem.com
What is Zinc Chloride and How to Buy Zinc Chloride? Zinc Chloride Hydrate Formula The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Hydrates generally contain water in stoichiometric amounts; Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: The five known hydrates. Zinc Chloride Hydrate Formula.
From www.indiamart.com
250 g Zinc Chloride Dry at best price in Pinjore ID 23826350562 Zinc Chloride Hydrate Formula Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Data from nist standard reference database 69: The formula of a hydrate can be determined by dehydrating a. Zinc Chloride Hydrate Formula.
From www.pw.live
Zinc Chloride Formula Zinc Chloride Hydrate Formula 1) determine the percentage of water in cobalt(ii) chloride dihydrate: Hydrates generally contain water in stoichiometric amounts; Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Data from nist standard reference database 69: Zinc chlorides, of which at nine crystalline forms are. The five known hydrates of zinc chloride have the general formula zncl 2. Zinc Chloride Hydrate Formula.
From www.flinnsci.com
Zinc Chloride, 500 g Flinn Scientific Zinc Chloride Hydrate Formula Data from nist standard reference database 69: Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of. Zinc Chloride Hydrate Formula.
From dir.indiamart.com
Zinc Chloride ZnCl2 Latest Price, Manufacturers & Suppliers Zinc Chloride Hydrate Formula Nist chemistry webbook the national institute of standards and technology (nist) uses its best. Zinc chloride is the name of chemical compound with the formula zn cl 2 and its hydrates. The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Hydrates generally contain water in. Zinc Chloride Hydrate Formula.
From pubs.acs.org
Crystalline and Liquid Structure of Zinc Chloride Trihydrate A Unique Zinc Chloride Hydrate Formula 1) determine the percentage of water in cobalt(ii) chloride dihydrate: The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5, 2.5, 3, and 4. Solid zncl 2 exhibits polymorphism and can take on one of the following crystal structures: Data from nist standard reference. Zinc Chloride Hydrate Formula.
From ramonnewscook.blogspot.com
Zinc Nitrate + Sodium Carbonate Balanced Equation Zinc Chloride Hydrate Formula Data from nist standard reference database 69: Nist chemistry webbook the national institute of standards and technology (nist) uses its best. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. Solid zncl 2 exhibits polymorphism. Zinc Chloride Hydrate Formula.
From cymitquimica.com
Zinc Chloride (High Purity & Low water content) 3BZ0053 Zinc Chloride Hydrate Formula The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate. 1) determine the percentage of water in cobalt(ii) chloride dihydrate: The five known hydrates of zinc chloride have the general formula zncl 2 (h 2 o) n where the values of n can be 1, 1.5,. Zinc Chloride Hydrate Formula.