Temperature Change Graph Chemistry . 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Plot a graph of your results and record the temperature change Molecules only react if they have sufficient energy for a reaction to take place. The temperature change will vary depending on the. When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. Measure the temperature changes during the reaction; Temperature change (neutralisation) 7 july 2020. Temperature changes in reacting solutions. To perform a calorimetry study of the reaction between hcl and naoh.
from ch301.cm.utexas.edu
To perform a calorimetry study of the reaction between hcl and naoh. Plot a graph of your results and record the temperature change This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. The temperature change will vary depending on the. Measure the temperature changes during the reaction; Temperature changes in reacting solutions. When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. Temperature change (neutralisation) 7 july 2020. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. Molecules only react if they have sufficient energy for a reaction to take place.
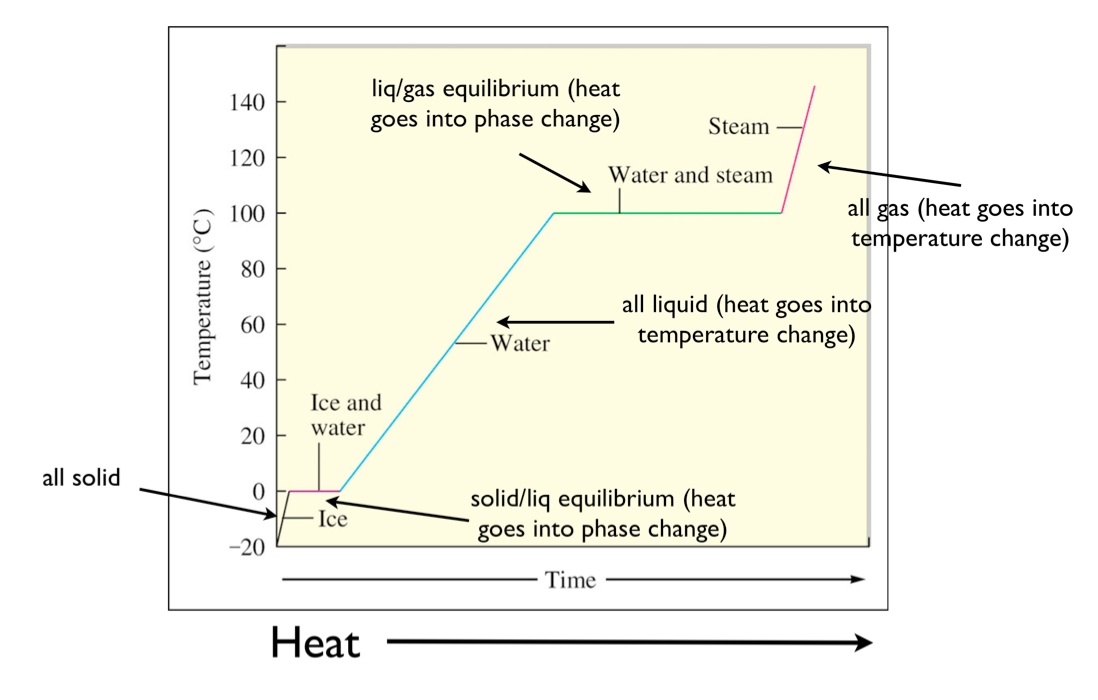
heating curve
Temperature Change Graph Chemistry Molecules only react if they have sufficient energy for a reaction to take place. When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. Molecules only react if they have sufficient energy for a reaction to take place. Measure the temperature changes during the reaction; 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. To perform a calorimetry study of the reaction between hcl and naoh. Plot a graph of your results and record the temperature change The temperature change will vary depending on the. Temperature change (neutralisation) 7 july 2020. Temperature changes in reacting solutions. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Temperature Change Graph Chemistry When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Plot a graph of your results and record the temperature change Temperature change (neutralisation). Temperature Change Graph Chemistry.
From www.slideshare.net
CHEMICAL REACTIONS Temperature Change Graph Chemistry Measure the temperature changes during the reaction; The temperature change will vary depending on the. Molecules only react if they have sufficient energy for a reaction to take place. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. To. Temperature Change Graph Chemistry.
From chem.libretexts.org
5.5.1 Heating Curves and Phase Changes (Problems) Chemistry LibreTexts Temperature Change Graph Chemistry This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. To perform a calorimetry study of the reaction between hcl and naoh. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m =. Temperature Change Graph Chemistry.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Temperature Change Graph Chemistry Molecules only react if they have sufficient energy for a reaction to take place. Temperature change (neutralisation) 7 july 2020. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Plot a graph of your results and record the temperature. Temperature Change Graph Chemistry.
From www.chemistrystudent.com
Boltzmann Distribution Curves (ALevel) ChemistryStudent Temperature Change Graph Chemistry 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. Temperature changes in reacting solutions. Molecules only react if they have sufficient energy for a reaction to take place. When the temperature of a solution increases, the molecular energy levels. Temperature Change Graph Chemistry.
From opentextbc.ca
PressurevsTemperature21 Introductory Chemistry 1st Canadian Edition Temperature Change Graph Chemistry Measure the temperature changes during the reaction; The temperature change will vary depending on the. To perform a calorimetry study of the reaction between hcl and naoh. Plot a graph of your results and record the temperature change When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. 1) δt = the change in. Temperature Change Graph Chemistry.
From courses.lumenlearning.com
Phase Diagrams Chemistry for Majors Temperature Change Graph Chemistry Plot a graph of your results and record the temperature change 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. The temperature change will vary depending on the. To perform a calorimetry study of the reaction between hcl and. Temperature Change Graph Chemistry.
From jackwestin.com
Phase Diagram Pressure And Temperature Energy Changes In Chemical Temperature Change Graph Chemistry Measure the temperature changes during the reaction; Plot a graph of your results and record the temperature change To perform a calorimetry study of the reaction between hcl and naoh. When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. Temperature changes in reacting solutions. Molecules only react if they have sufficient energy for. Temperature Change Graph Chemistry.
From socratic.org
How do graph temperature versus time for a pure substance? Socratic Temperature Change Graph Chemistry Molecules only react if they have sufficient energy for a reaction to take place. Measure the temperature changes during the reaction; This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Plot a graph of your results and record the. Temperature Change Graph Chemistry.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Temperature Change Graph Chemistry Molecules only react if they have sufficient energy for a reaction to take place. To perform a calorimetry study of the reaction between hcl and naoh. The temperature change will vary depending on the. Temperature change (neutralisation) 7 july 2020. When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. This is the case. Temperature Change Graph Chemistry.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Temperature Change Graph Chemistry The temperature change will vary depending on the. Plot a graph of your results and record the temperature change Molecules only react if they have sufficient energy for a reaction to take place. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p =. Temperature Change Graph Chemistry.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Temperature Change Graph Chemistry Temperature changes in reacting solutions. When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. To perform a calorimetry study of the reaction between hcl and naoh. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p =. Temperature Change Graph Chemistry.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes Chemistry LibreTexts Temperature Change Graph Chemistry When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. To perform a calorimetry study of the reaction between hcl and naoh. Molecules only react if they have sufficient energy for a reaction to take place. Measure the temperature changes during the reaction; 1) δt = the change in temperature from start to finish. Temperature Change Graph Chemistry.
From www.worldwisetutoring.com
Heating and Cooling Curves Temperature Change Graph Chemistry To perform a calorimetry study of the reaction between hcl and naoh. When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. The temperature change will vary depending on the. Temperature changes in reacting solutions. Temperature change (neutralisation) 7 july 2020. Measure the temperature changes during the reaction; 1) δt = the change in. Temperature Change Graph Chemistry.
From courses.lumenlearning.com
Solid to Gas Phase Transition Introduction to Chemistry Temperature Change Graph Chemistry 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. Temperature changes in reacting solutions. Molecules only react if they have sufficient energy for a reaction to take place. The temperature change will vary depending on the. Temperature change (neutralisation). Temperature Change Graph Chemistry.
From askfilo.com
The graph below shows the heating curve for a pure substance. The tempera.. Temperature Change Graph Chemistry The temperature change will vary depending on the. Temperature changes in reacting solutions. Molecules only react if they have sufficient energy for a reaction to take place. Measure the temperature changes during the reaction; 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p. Temperature Change Graph Chemistry.
From nigerianscholars.com
Phase Changes Temperature, Theory, and Gas Laws Temperature Change Graph Chemistry This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Plot a graph of your results and record the temperature change Temperature change (neutralisation) 7 july 2020. To perform a calorimetry study of the reaction between hcl and naoh. When. Temperature Change Graph Chemistry.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Temperature Change Graph Chemistry Plot a graph of your results and record the temperature change Temperature change (neutralisation) 7 july 2020. To perform a calorimetry study of the reaction between hcl and naoh. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. When. Temperature Change Graph Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记4.1.4 Determining Enthalpy Change of Temperature Change Graph Chemistry 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. The temperature change will vary depending on the. When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. To perform a calorimetry study of. Temperature Change Graph Chemistry.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Temperature Change Graph Chemistry Temperature changes in reacting solutions. To perform a calorimetry study of the reaction between hcl and naoh. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. Molecules only react if they have sufficient energy for a reaction to take. Temperature Change Graph Chemistry.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Temperature Change Graph Chemistry To perform a calorimetry study of the reaction between hcl and naoh. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive. Temperature Change Graph Chemistry.
From www.researchgate.net
The temperaturevstime graph for the of H 2 O 2 (aq Temperature Change Graph Chemistry Temperature changes in reacting solutions. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Plot a graph of your results and record the temperature change Molecules only react if they have sufficient energy for a reaction to take place.. Temperature Change Graph Chemistry.
From chemistry101efhs.weebly.com
Heat vs. Temperature Chemistry 101 Temperature Change Graph Chemistry Molecules only react if they have sufficient energy for a reaction to take place. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Temperature change (neutralisation) 7 july 2020. 1) δt = the change in temperature from start to. Temperature Change Graph Chemistry.
From ch301.cm.utexas.edu
heating curve Temperature Change Graph Chemistry 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. Temperature change (neutralisation) 7 july 2020. Temperature changes in reacting solutions. Molecules only react if they have sufficient energy for a reaction to take place. Measure the temperature changes during. Temperature Change Graph Chemistry.
From www.youtube.com
How to Answer Equilibrium Graph Exam Questions // HSC Chemistry YouTube Temperature Change Graph Chemistry This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Temperature changes in reacting solutions. Measure the temperature changes during the reaction; Temperature change (neutralisation) 7 july 2020. Plot a graph of your results and record the temperature change The. Temperature Change Graph Chemistry.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Temperature Change Graph Chemistry Temperature change (neutralisation) 7 july 2020. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. Plot a graph of your results and record the temperature change Measure the temperature changes during the reaction; To perform a calorimetry study of. Temperature Change Graph Chemistry.
From tristennhuber.blogspot.com
Temperature Vs Time Graph TristennHuber Temperature Change Graph Chemistry Temperature change (neutralisation) 7 july 2020. The temperature change will vary depending on the. Temperature changes in reacting solutions. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Plot a graph of your results and record the temperature change. Temperature Change Graph Chemistry.
From www.dreamstime.com
Temperature Conversions Table. Converting between Celsius, Kelvin, and Temperature Change Graph Chemistry When the temperature of a solution increases, the molecular energy levels also increase, causing the reaction. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Molecules only react if they have sufficient energy for a reaction to take place.. Temperature Change Graph Chemistry.
From brainly.in
Q2. The given graph shows the heating curve for a pure substance,the Temperature Change Graph Chemistry Temperature changes in reacting solutions. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. The temperature change will vary depending on the. Temperature change (neutralisation) 7 july 2020. Measure the temperature changes during the reaction; Molecules only react if. Temperature Change Graph Chemistry.
From chart-studio.plotly.com
Effect of Temperature on Reaction Rate Using Magnesium and Hydrochloric Temperature Change Graph Chemistry This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Temperature change (neutralisation) 7 july 2020. Temperature changes in reacting solutions. Measure the temperature changes during the reaction; To perform a calorimetry study of the reaction between hcl and naoh.. Temperature Change Graph Chemistry.
From www.researchgate.net
Different heating temperature change graph (A) The heating temperature Temperature Change Graph Chemistry The temperature change will vary depending on the. Temperature changes in reacting solutions. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. Molecules only react if they have sufficient energy for a reaction to take place. Measure the temperature. Temperature Change Graph Chemistry.
From mungfali.com
Reaction Rate And Temperature Graph Temperature Change Graph Chemistry Temperature change (neutralisation) 7 july 2020. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. To perform a calorimetry study of the reaction between hcl and naoh. Plot a graph of your results and record the temperature change Molecules. Temperature Change Graph Chemistry.
From chemnotcheem.com
2019 O Level Pure Chemistry Practical Paper Chem Not Cheem Temperature Change Graph Chemistry To perform a calorimetry study of the reaction between hcl and naoh. This is the case for temperature conversions from fahrenheit to celsius, where both scales are measuring an intensive property, the temperature of some object or substance, and it is the. Plot a graph of your results and record the temperature change Temperature changes in reacting solutions. 1) δt. Temperature Change Graph Chemistry.
From www.shalom-education.com
Effect of Temperature on the Rate of Reaction GCSE Chemistry Revision Temperature Change Graph Chemistry Measure the temperature changes during the reaction; Temperature change (neutralisation) 7 july 2020. 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. Plot a graph of your results and record the temperature change This is the case for temperature. Temperature Change Graph Chemistry.
From chart-studio.plotly.com
A graph to show how the temperature affects the rate of reaction Temperature Change Graph Chemistry Molecules only react if they have sufficient energy for a reaction to take place. Temperature change (neutralisation) 7 july 2020. Measure the temperature changes during the reaction; 1) δt = the change in temperature from start to finish in degrees celsius (°c) 2) m = mass of substance in grams 3) c p = the specific heat. When the temperature. Temperature Change Graph Chemistry.