Titration Curve Report . Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Here are reduced versions of. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. A summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. A titration curve is a plot of some solution property versus the amount of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong.
from www.studocu.com
The figure below shows two different examples of a strong. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. Here are reduced versions of. A summary of the important curves. A titration curve is a plot of some solution property versus the amount of added titrant. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. The way you normally carry out a titration involves adding the acid to the alkali. A titration curve is a graphical representation of the ph of a solution during a titration. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization.
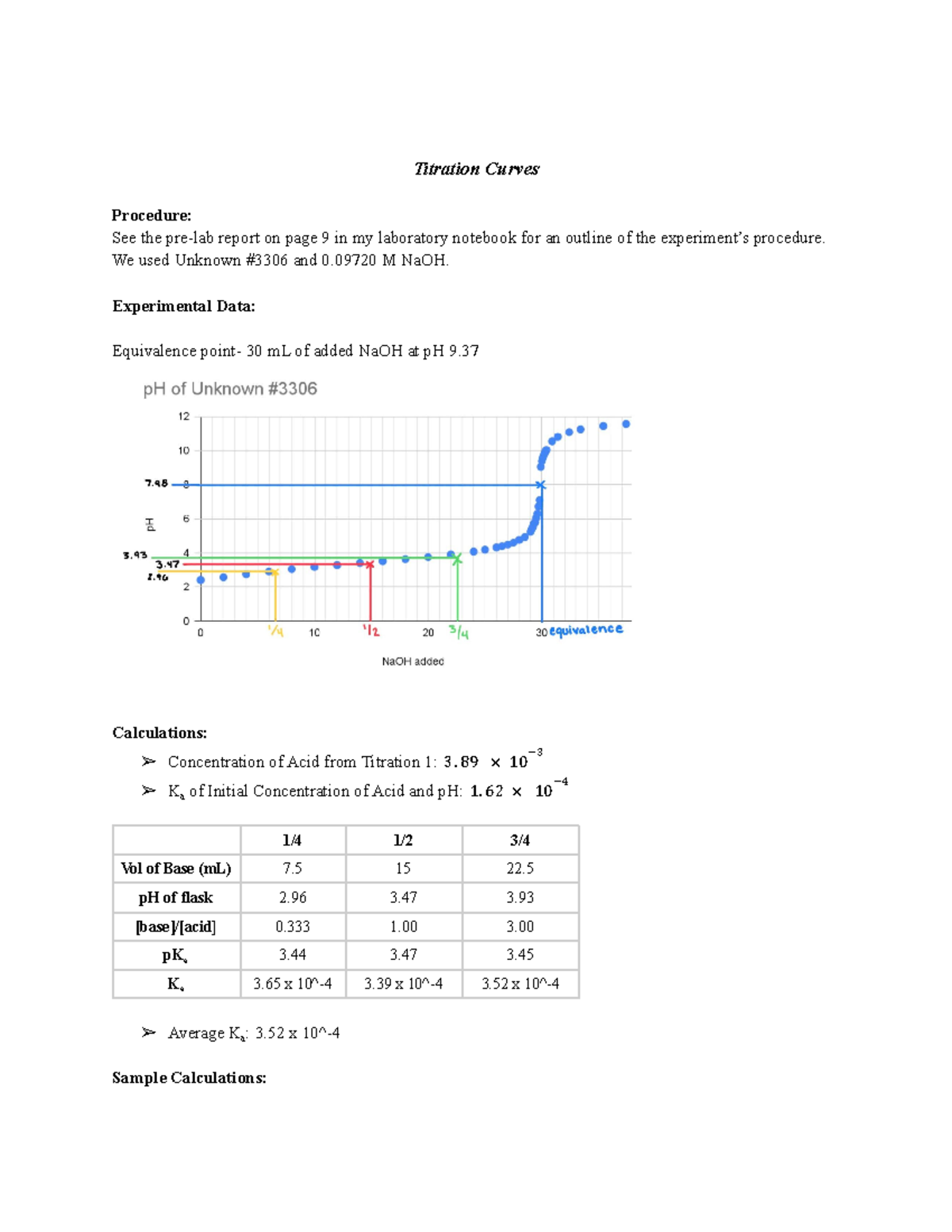
Titration Curves Titration Curves Procedure See the prelab report
Titration Curve Report A titration curve is a graphical representation of the ph of a solution during a titration. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The figure below shows two different examples of a strong. Here are reduced versions of. A summary of the important curves. A titration curve is a graphical representation of the ph of a solution during a titration. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. The way you normally carry out a titration involves adding the acid to the alkali. A titration curve is a plot of some solution property versus the amount of added titrant.
From www.youtube.com
TRU Chemistry labs How To Plot a Titration Curve YouTube Titration Curve Report Here are reduced versions of. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong.. Titration Curve Report.
From www.studocu.com
Lab Report 15 Titration Curves “Drop the base” Titration Curves Titration Curve Report A summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration curve is a plot of some solution property versus the amount of added titrant. In a ph titration you measure the. Titration Curve Report.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Curve Report A titration curve is a graphical representation of the ph of a solution during a titration. A summary of the important curves. Here are reduced versions of. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in. Titration Curve Report.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Report A summary of the important curves. The way you normally carry out a titration involves adding the acid to the alkali. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. A titration curve. Titration Curve Report.
From www.solutionspile.com
[Solved] Determine the equivalent volume for the followin Titration Curve Report A titration curve is a plot of some solution property versus the amount of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The figure below shows two different examples of a strong. Here are. Titration Curve Report.
From biochemden.com
Titration Curve of Glycine The zwitter ionic changes Titration Curve Report The way you normally carry out a titration involves adding the acid to the alkali. A titration curve is a graphical representation of the ph of a solution during a titration. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is.. Titration Curve Report.
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Curve Report A summary of the important curves. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Learn all about titration analysis, including titration curves &. Titration Curve Report.
From www.youtube.com
17.3 Weak Acid Strong Base Titration Curve YouTube Titration Curve Report Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. A titration curve is a graphical representation of the ph of a solution during a titration. A summary of the important curves. Here are. Titration Curve Report.
From www.slideserve.com
PPT Acidity Constant by pH Titration Curves PowerPoint Presentation Titration Curve Report A titration curve is a graphical representation of the ph of a solution during a titration. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Here are reduced versions of. The figure below shows two different examples of a strong. The way you normally carry out a titration involves adding the acid to. Titration Curve Report.
From solvedlib.com
The graph below shows the titration curves for two mo… SolvedLib Titration Curve Report A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. The way you normally carry out a titration involves adding the acid to the alkali. A titration curve is a plot of some solution property versus the amount of added titrant. Learn all about. Titration Curve Report.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Report Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration curve is a graphical representation of the ph of a solution during a titration. Here are reduced versions of. The way you normally carry out a titration involves adding the acid to the alkali. Learn all about titration analysis, including titration curves. Titration Curve Report.
From www.studocu.com
Gonzalez D Titration Curve Lab Report Dayanyra Gonzalez 06/11 Titration Curve Report Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. A titration curve is a plot of some solution property versus the amount of added titrant. The way you normally carry out a titration involves adding the acid to the alkali. In a ph titration you measure the ph as. Titration Curve Report.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Report In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. A titration curve is a plot of some solution property versus the amount of added titrant. A titration curve is a graphical representation of the ph of a solution during a. Titration Curve Report.
From courses.lumenlearning.com
15.2 AcidBase Titrations Chemistry Titration Curve Report Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Here are reduced versions of. The way you normally carry out a titration involves adding the acid to the alkali. The figure below shows two different examples of a strong. A titration curve is a plot of some solution property versus the amount of. Titration Curve Report.
From www.researchgate.net
Potentiometric back titration of INH. Download Scientific Diagram Titration Curve Report In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. A titration curve is a plot of some solution property versus the amount of added titrant. Here are reduced versions of. A summary of the important curves. The figure below shows. Titration Curve Report.
From es.scribd.com
Titration Curve Titration Chemistry Titration Curve Report A titration curve is a graphical representation of the ph of a solution during a titration. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. The figure below shows two different examples of a strong. Determine the concentration of analyte present, as well as the acid ionization constant and. Titration Curve Report.
From loeigruoo.blob.core.windows.net
How To Do A Titration Graph at Terry Bailey blog Titration Curve Report Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The way you normally carry out a titration involves adding the acid to the alkali. A titration curve is a plot of some solution property versus the amount of added titrant. A titration curve is a graphical representation of the ph of a solution. Titration Curve Report.
From chart-studio.plotly.com
Titration Curve for Selected Buffers scatter chart made by Lucastrim5 Titration Curve Report In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. The figure below shows two different examples of a strong. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. A. Titration Curve Report.
From www.writework.com
Titration of amino acids WriteWork Titration Curve Report The figure below shows two different examples of a strong. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration curve is a. Titration Curve Report.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Report The way you normally carry out a titration involves adding the acid to the alkali. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a. Titration Curve Report.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Report The way you normally carry out a titration involves adding the acid to the alkali. The figure below shows two different examples of a strong. A summary of the important curves. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. Determine the concentration of analyte present, as well as. Titration Curve Report.
From mungfali.com
Endpoint Titration Curve Titration Curve Report In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The way you normally carry out a titration involves adding the acid to the alkali.. Titration Curve Report.
From www.studypool.com
SOLUTION Titration Curve 2 Studypool Titration Curve Report The figure below shows two different examples of a strong. The way you normally carry out a titration involves adding the acid to the alkali. Here are reduced versions of. A summary of the important curves. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration curve is a plot of some. Titration Curve Report.
From www.researchgate.net
Titration data (9) already shown in Figure 2 are here reported on the Titration Curve Report The way you normally carry out a titration involves adding the acid to the alkali. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. Here are reduced versions of. A titration curve is a plot of some solution property versus the amount of added titrant. A summary of the. Titration Curve Report.
From www.studocu.com
Titration Curves Lab Report Experiment 5 Acid/Base Titration Curves Titration Curve Report The figure below shows two different examples of a strong. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A titration curve is a plot of some solution property versus the amount of added titrant. The way you normally carry out a titration involves adding the acid to the alkali. A summary of. Titration Curve Report.
From www.youtube.com
Excel Tutorial 2 Titration Analysis YouTube Titration Curve Report A titration curve is a graphical representation of the ph of a solution during a titration. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. The way you normally carry out a titration involves adding the acid to the alkali. A summary of the important curves. In a ph. Titration Curve Report.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve Report The figure below shows two different examples of a strong. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. The way you normally carry out a titration involves adding the acid to the. Titration Curve Report.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Curve Report A titration curve is a graphical representation of the ph of a solution during a titration. Here are reduced versions of. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. A summary of. Titration Curve Report.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Report In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. The figure below shows two different examples of a strong. Determine. Titration Curve Report.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Report In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. The figure below shows two different examples of a strong. Here are reduced versions of. The way you normally carry out a titration involves adding the acid to the alkali. A. Titration Curve Report.
From narodnatribuna.info
Lab 8 Titration Curves Titration Curve Report The way you normally carry out a titration involves adding the acid to the alkali. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. The figure below shows two different examples of a strong. A titration curve is a plot. Titration Curve Report.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Report A titration curve is a plot of some solution property versus the amount of added titrant. The figure below shows two different examples of a strong. Here are reduced versions of. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is.. Titration Curve Report.
From pubs.sciepub.com
Figure 5B. Plot of the titration of strong acid (HCl= 0.1M) with strong Titration Curve Report The way you normally carry out a titration involves adding the acid to the alkali. Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. A summary of the important curves. Here are reduced versions of. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the. Titration Curve Report.
From www.researchgate.net
The realtime titration curve of MA Download Scientific Diagram Titration Curve Report Determine the concentration of analyte present, as well as the acid ionization constant and base ionization. In a ph titration you measure the ph as a function of the volume of titrant added and determine the equivalence point as the point in where there is. A titration curve is a plot of some solution property versus the amount of added. Titration Curve Report.
From www.studocu.com
Titration Curves Titration Curves Procedure See the prelab report Titration Curve Report The way you normally carry out a titration involves adding the acid to the alkali. Learn all about titration analysis, including titration curves & calculations, equivalence point, & steps of titrations in the chemistry lab. A summary of the important curves. In a ph titration you measure the ph as a function of the volume of titrant added and determine. Titration Curve Report.