Models Of Covalent Bonding . the valence bond theory is introduced to describe bonding in covalent molecules. how does electron sharing lead to bonding between atoms? Lewis in 1916, and it remains the most widely. In this model, bonds are. Bonding between a metal and a nonmetal is often ionic. bonds between two nonmetals are generally covalent; For each molecule, there are different names for pairs of electrons, depending if it is shared or not. This model originated with the theory developed by g.n. A pair of electrons that is shared between two atoms is called a bond pair. Two models have been developed to describe covalent bonding: the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form.
from www.worksheetsplanet.com
A pair of electrons that is shared between two atoms is called a bond pair. Two models have been developed to describe covalent bonding: In this model, bonds are. how does electron sharing lead to bonding between atoms? Bonding between a metal and a nonmetal is often ionic. bonds between two nonmetals are generally covalent; Lewis in 1916, and it remains the most widely. This model originated with the theory developed by g.n. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. For each molecule, there are different names for pairs of electrons, depending if it is shared or not.
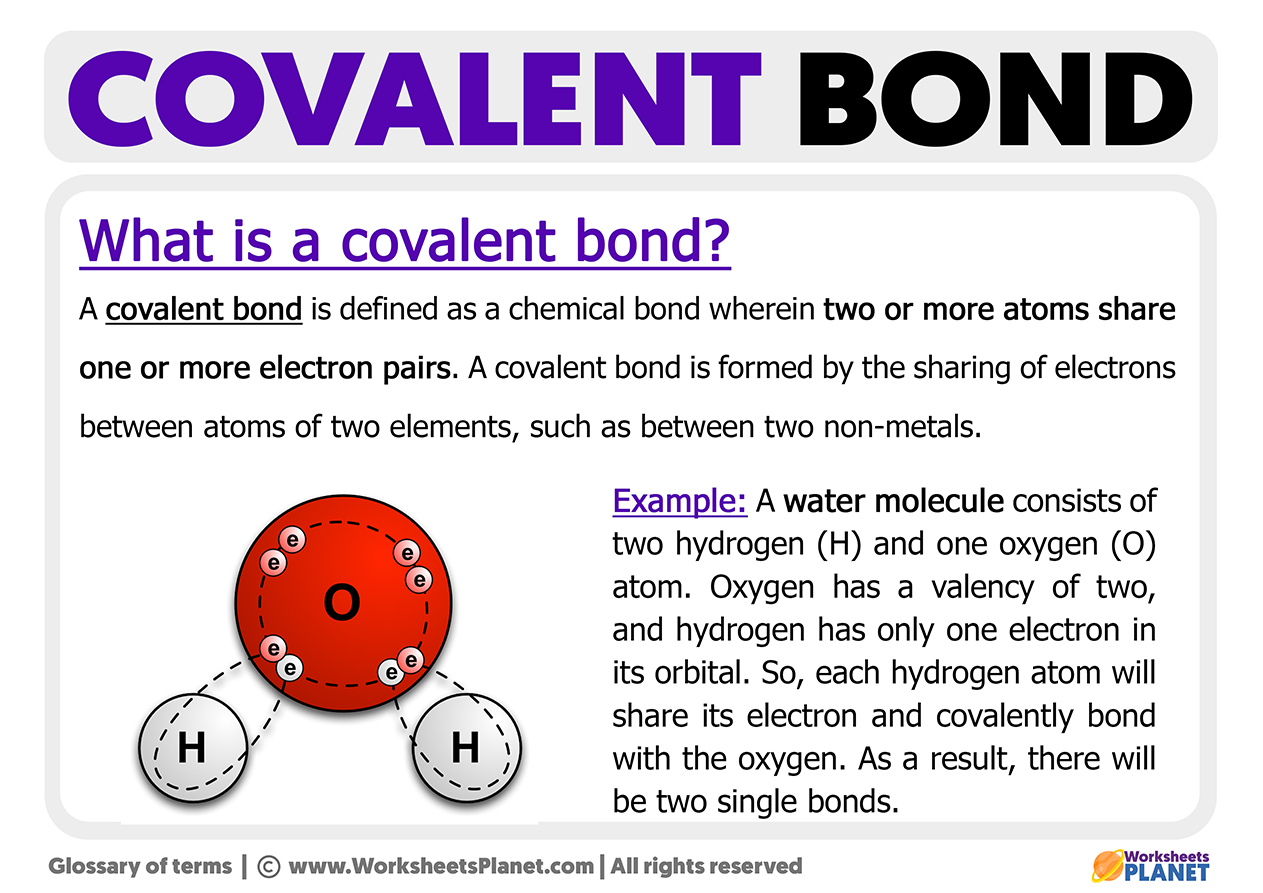
What is a Covalent Bond?
Models Of Covalent Bonding the valence bond theory is introduced to describe bonding in covalent molecules. A pair of electrons that is shared between two atoms is called a bond pair. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. In this model, bonds are. bonds between two nonmetals are generally covalent; Two models have been developed to describe covalent bonding: the valence bond theory is introduced to describe bonding in covalent molecules. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. Lewis in 1916, and it remains the most widely. Bonding between a metal and a nonmetal is often ionic. This model originated with the theory developed by g.n. how does electron sharing lead to bonding between atoms?
From emedia.rmit.edu.au
Covalent bonds Learning Lab Models Of Covalent Bonding A pair of electrons that is shared between two atoms is called a bond pair. This model originated with the theory developed by g.n. Two models have been developed to describe covalent bonding: Lewis in 1916, and it remains the most widely. For each molecule, there are different names for pairs of electrons, depending if it is shared or not.. Models Of Covalent Bonding.
From sciencenotes.org
Covalent Compounds Examples and Properties Models Of Covalent Bonding Lewis in 1916, and it remains the most widely. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. the valence bond theory is introduced to describe bonding in covalent molecules. Two models have been developed to describe covalent bonding: essential understanding scientists use a variety of theories and. Models Of Covalent Bonding.
From sketchfab.com
Triple Covalent Bond 3D model by Andy Todd (atodd19) [da26450 Models Of Covalent Bonding essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. Lewis in 1916, and it remains the most widely. the valence bond theory is introduced to describe bonding in covalent molecules. how does electron sharing lead to bonding between atoms? Bonding between a metal and a nonmetal is often. Models Of Covalent Bonding.
From chem.libretexts.org
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts Models Of Covalent Bonding This model originated with the theory developed by g.n. bonds between two nonmetals are generally covalent; the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. how does electron sharing lead to bonding between atoms? Two models have been developed to describe covalent bonding: Bonding between a metal and. Models Of Covalent Bonding.
From www.pinterest.com.mx
Chemistry Lab Molecular Models of Covalent Compounds (Polar or Models Of Covalent Bonding essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. In this model, bonds are. bonds between two nonmetals are generally covalent; For each molecule, there are different names for pairs of electrons, depending if it is shared or not. how does electron sharing lead to bonding between atoms?. Models Of Covalent Bonding.
From www.vrogue.co
7 Awesome Covalent Bond 3d Model You Mockup vrogue.co Models Of Covalent Bonding In this model, bonds are. essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. how does electron sharing lead to bonding between atoms? Two models have been developed to describe. Models Of Covalent Bonding.
From saylordotorg.github.io
Molecular Geometry and Covalent Bonding Models Models Of Covalent Bonding In this model, bonds are. A pair of electrons that is shared between two atoms is called a bond pair. the valence bond theory is introduced to describe bonding in covalent molecules. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. Bonding between a metal and a nonmetal is often. Models Of Covalent Bonding.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Models Of Covalent Bonding how does electron sharing lead to bonding between atoms? the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. For each molecule, there are different names for pairs of electrons, depending. Models Of Covalent Bonding.
From www.thoughtco.com
Examples of Covalent Bonds and Compounds Models Of Covalent Bonding how does electron sharing lead to bonding between atoms? Bonding between a metal and a nonmetal is often ionic. This model originated with the theory developed by g.n. the valence bond theory is introduced to describe bonding in covalent molecules. Two models have been developed to describe covalent bonding: A pair of electrons that is shared between two. Models Of Covalent Bonding.
From chem.libretexts.org
8.7 Bond Polarity and Electronegativity (\(\chi\)) Chemistry LibreTexts Models Of Covalent Bonding In this model, bonds are. Bonding between a metal and a nonmetal is often ionic. bonds between two nonmetals are generally covalent; the valence bond theory is introduced to describe bonding in covalent molecules. Two models have been developed to describe covalent bonding: essential understanding scientists use a variety of theories and models to explain how and. Models Of Covalent Bonding.
From www.worksheetsplanet.com
What is a Covalent Bond? Models Of Covalent Bonding essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. bonds between two nonmetals are generally covalent; the valence bond theory is introduced to describe bonding in covalent molecules. A pair of electrons that is shared between two atoms is called a bond pair. Two models have been developed. Models Of Covalent Bonding.
From www.youtube.com
MODEL OF COVALENT COMPOUNDS(COVALENT BONDING))CLASS TENTHSCIENCE Models Of Covalent Bonding Lewis in 1916, and it remains the most widely. the valence bond theory is introduced to describe bonding in covalent molecules. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. This model originated with the theory developed by g.n. bonds between two nonmetals are generally covalent; In this. Models Of Covalent Bonding.
From saylordotorg.github.io
Molecular Geometry and Covalent Bonding Models Models Of Covalent Bonding Bonding between a metal and a nonmetal is often ionic. This model originated with the theory developed by g.n. In this model, bonds are. how does electron sharing lead to bonding between atoms? Two models have been developed to describe covalent bonding: the types of covalent bonds can be distinguished by looking at the lewis dot structure of. Models Of Covalent Bonding.
From overallscience.com
Covalent bond (covalency) and its type Overall Science Models Of Covalent Bonding In this model, bonds are. bonds between two nonmetals are generally covalent; how does electron sharing lead to bonding between atoms? For each molecule, there are different names for pairs of electrons, depending if it is shared or not. the valence bond theory is introduced to describe bonding in covalent molecules. essential understanding scientists use a. Models Of Covalent Bonding.
From www.cgtrader.com
3D model Covalent bond h2o VR / AR / lowpoly CGTrader Models Of Covalent Bonding Two models have been developed to describe covalent bonding: This model originated with the theory developed by g.n. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. essential understanding scientists use. Models Of Covalent Bonding.
From www.sliderbase.com
Covalent Bonding (Molecules) Presentation Chemistry Models Of Covalent Bonding A pair of electrons that is shared between two atoms is called a bond pair. Lewis in 1916, and it remains the most widely. Bonding between a metal and a nonmetal is often ionic. This model originated with the theory developed by g.n. bonds between two nonmetals are generally covalent; Two models have been developed to describe covalent bonding:. Models Of Covalent Bonding.
From sansona.github.io
Attractive Forces and Bonds Models Of Covalent Bonding This model originated with the theory developed by g.n. essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. bonds between two nonmetals are generally covalent; the valence bond theory. Models Of Covalent Bonding.
From www.youtube.com
Covalent Bonding DotCross Diagrams GCSE Chemistry Revision YouTube Models Of Covalent Bonding the valence bond theory is introduced to describe bonding in covalent molecules. bonds between two nonmetals are generally covalent; Lewis in 1916, and it remains the most widely. This model originated with the theory developed by g.n. In this model, bonds are. Bonding between a metal and a nonmetal is often ionic. For each molecule, there are different. Models Of Covalent Bonding.
From oerpub.github.io
The top panel in this figure shows two hydrogen atoms sharing two Models Of Covalent Bonding essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. Two models have been developed to describe covalent bonding: This model originated with the theory developed by g.n. the valence bond theory is introduced to describe bonding in covalent molecules. bonds between two nonmetals are generally covalent; In this. Models Of Covalent Bonding.
From www.britannica.com
Covalent bond Definition, Properties, Examples, & Facts Britannica Models Of Covalent Bonding Lewis in 1916, and it remains the most widely. the valence bond theory is introduced to describe bonding in covalent molecules. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. Bonding between a metal and a nonmetal is often ionic. A pair of electrons that is shared between two. Models Of Covalent Bonding.
From www.thesciencehive.co.uk
Covalent bonding (GCSE) — the science sauce Models Of Covalent Bonding bonds between two nonmetals are generally covalent; Lewis in 1916, and it remains the most widely. Bonding between a metal and a nonmetal is often ionic. how does electron sharing lead to bonding between atoms? the valence bond theory is introduced to describe bonding in covalent molecules. This model originated with the theory developed by g.n. . Models Of Covalent Bonding.
From biologydictionary.net
Covalent Bond Biology Dictionary Models Of Covalent Bonding essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. Two models have been developed to describe covalent bonding: Lewis in 1916, and it remains the most widely. Bonding between a metal and. Models Of Covalent Bonding.
From mavink.com
Covalent Bond Types Models Of Covalent Bonding how does electron sharing lead to bonding between atoms? the valence bond theory is introduced to describe bonding in covalent molecules. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. For each molecule, there are different names for pairs of electrons, depending if it is shared or not.. Models Of Covalent Bonding.
From mavink.com
Polar Covalent Bond Model Models Of Covalent Bonding For each molecule, there are different names for pairs of electrons, depending if it is shared or not. A pair of electrons that is shared between two atoms is called a bond pair. In this model, bonds are. Bonding between a metal and a nonmetal is often ionic. essential understanding scientists use a variety of theories and models to. Models Of Covalent Bonding.
From sciencestruck.com
ScienceStruck Models Of Covalent Bonding bonds between two nonmetals are generally covalent; essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. how does electron sharing lead to bonding between atoms? Lewis in 1916, and it remains the most widely. This model originated with the theory developed by g.n. the types of covalent. Models Of Covalent Bonding.
From clarence-well-duffy.blogspot.com
Explain the Major Differences Between Covalent and Ionic Bonding Models Of Covalent Bonding A pair of electrons that is shared between two atoms is called a bond pair. Two models have been developed to describe covalent bonding: the valence bond theory is introduced to describe bonding in covalent molecules. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. the types of covalent. Models Of Covalent Bonding.
From ar.inspiredpencil.com
Covalent Bond Examples Models Of Covalent Bonding Lewis in 1916, and it remains the most widely. A pair of electrons that is shared between two atoms is called a bond pair. essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. Bonding between a metal and a nonmetal is often ionic. This model originated with the theory developed. Models Of Covalent Bonding.
From www.britannica.com
crystal Types of bonds Britannica Models Of Covalent Bonding how does electron sharing lead to bonding between atoms? the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. Bonding between a metal and a nonmetal is often ionic. In this model,. Models Of Covalent Bonding.
From ar.inspiredpencil.com
Ionic Bond 3d Model Models Of Covalent Bonding the valence bond theory is introduced to describe bonding in covalent molecules. Two models have been developed to describe covalent bonding: essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. . Models Of Covalent Bonding.
From www.vrogue.co
19 Awesome Covalent Bond 3d Model Project Emgold Mock vrogue.co Models Of Covalent Bonding how does electron sharing lead to bonding between atoms? In this model, bonds are. bonds between two nonmetals are generally covalent; the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. A pair of electrons that is shared between two atoms is called a bond pair. For each molecule,. Models Of Covalent Bonding.
From saylordotorg.github.io
Molecular Geometry and Covalent Bonding Models Models Of Covalent Bonding how does electron sharing lead to bonding between atoms? the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. bonds between two nonmetals are generally covalent; Bonding between a metal and a nonmetal is often ionic. For each molecule, there are different names for pairs of electrons, depending if. Models Of Covalent Bonding.
From mmerevise.co.uk
Covalent Bonding Questions and Revision MME Models Of Covalent Bonding Bonding between a metal and a nonmetal is often ionic. This model originated with the theory developed by g.n. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. Two models have been. Models Of Covalent Bonding.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Models Of Covalent Bonding This model originated with the theory developed by g.n. the types of covalent bonds can be distinguished by looking at the lewis dot structure of the molecule. In this model, bonds are. essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. bonds between two nonmetals are generally covalent;. Models Of Covalent Bonding.
From courses.lumenlearning.com
Reading Covalent Bonds Biology I Models Of Covalent Bonding Bonding between a metal and a nonmetal is often ionic. essential understanding scientists use a variety of theories and models to explain how and why covalent bonds form. how does electron sharing lead to bonding between atoms? A pair of electrons that is shared between two atoms is called a bond pair. In this model, bonds are. Two. Models Of Covalent Bonding.
From www.tec-science.com
Covalent bonding tecscience Models Of Covalent Bonding Two models have been developed to describe covalent bonding: the valence bond theory is introduced to describe bonding in covalent molecules. For each molecule, there are different names for pairs of electrons, depending if it is shared or not. Bonding between a metal and a nonmetal is often ionic. This model originated with the theory developed by g.n. In. Models Of Covalent Bonding.