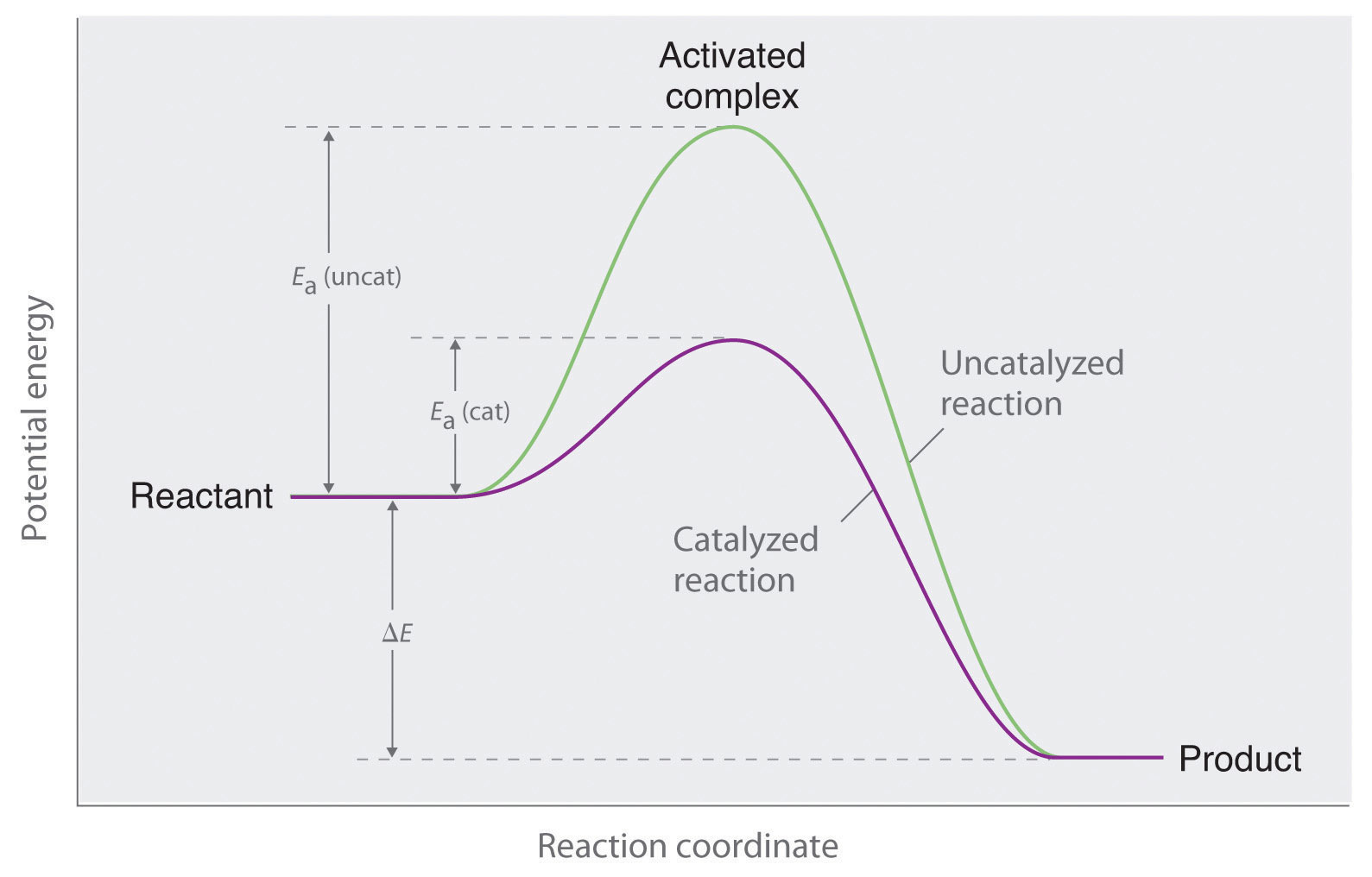
Catalytic Reaction Meaning . catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. Learn about homogeneous and heterogeneous. a catalyst is a substance that speeds up a chemical reaction without being used up. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about the characteristics, types, and. Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about different types of catalysts, how they work, and their. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. a catalyst is a substance that alters the rate of a reaction without being consumed.
from 2012books.lardbucket.org
a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. a catalyst is a substance that alters the rate of a reaction without being consumed. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about homogeneous and heterogeneous. Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn about different types of catalysts, how they work, and their. a catalyst is a substance that speeds up a chemical reaction without being used up. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about the characteristics, types, and.
Catalysis
Catalytic Reaction Meaning a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. Learn about homogeneous and heterogeneous. a catalyst is a substance that alters the rate of a reaction without being consumed. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. Learn about different types of catalysts, how they work, and their. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about the characteristics, types, and. a catalyst is a substance that speeds up a chemical reaction without being used up.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalytic Reaction Meaning Learn about different types of catalysts, how they work, and their. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. catalysis is the modification of the rate of a chemical reaction by addition of. Catalytic Reaction Meaning.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalytic Reaction Meaning a catalyst is a substance that alters the rate of a reaction without being consumed. Learn about different types of catalysts, how they work, and their. Learn about homogeneous and heterogeneous. Learn about the characteristics, types, and. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn how catalysts work, types of catalysts,. Catalytic Reaction Meaning.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalytic Reaction Meaning Learn about the characteristics, types, and. Learn about homogeneous and heterogeneous. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. a catalyst is a substance that speeds up a chemical reaction without being used up. Learn. Catalytic Reaction Meaning.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Catalytic Reaction Meaning Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. Learn about the characteristics, types, and. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn. Catalytic Reaction Meaning.
From chem.libretexts.org
8.1 Catalytic reactions Chemistry LibreTexts Catalytic Reaction Meaning Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. a catalyst is a substance that alters the rate of a reaction without being consumed. Learn about the characteristics, types, and. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about homogeneous and heterogeneous. . Catalytic Reaction Meaning.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalytic Reaction Meaning a catalyst is a substance that speeds up a chemical reaction without being used up. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about the characteristics, types, and. Learn about the different types of. Catalytic Reaction Meaning.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalytic Reaction Meaning Learn about the characteristics, types, and. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. . Catalytic Reaction Meaning.
From www.slideserve.com
PPT Heterogeneous catalysis PowerPoint Presentation, free download Catalytic Reaction Meaning Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about homogeneous and heterogeneous. Learn about different types of catalysts, how they work, and their. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. a catalyst is a substance that affects the. Catalytic Reaction Meaning.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalytic Reaction Meaning a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. a catalyst is a substance that speeds up a chemical reaction without being used up. Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. a catalyst is a substance. Catalytic Reaction Meaning.
From exyejvooa.blob.core.windows.net
Catalyst Definition With Examples at Aurelia Martin blog Catalytic Reaction Meaning a catalyst is a substance that speeds up a chemical reaction without being used up. a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. Learn how catalysts. Catalytic Reaction Meaning.
From 2012books.lardbucket.org
Catalysis Catalytic Reaction Meaning Learn about different types of catalysts, how they work, and their. a catalyst is a substance that speeds up a chemical reaction without being used up. a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. a catalyst is a substance that alters the rate of a reaction without. Catalytic Reaction Meaning.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Catalytic Reaction Meaning a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. a catalyst is a substance that speeds up a chemical reaction without being used up. Learn about homogeneous and heterogeneous. Learn about the characteristics, types, and. Learn about different types of catalysts, how they work, and their. Learn how catalysts. Catalytic Reaction Meaning.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalytic Reaction Meaning Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn about the characteristics, types, and. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during. Catalytic Reaction Meaning.
From www.alamy.com
A diagram showing the catalytic reaction the energy niveau as a Catalytic Reaction Meaning Learn about different types of catalysts, how they work, and their. Learn about homogeneous and heterogeneous. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. a catalyst is a substance that. Catalytic Reaction Meaning.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalytic Reaction Meaning Learn about homogeneous and heterogeneous. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. a catalyst is a substance that speeds up a chemical reaction without being used up. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during. Catalytic Reaction Meaning.
From exyvshuta.blob.core.windows.net
Catalyst In Chemistry Example at Sharon Kane blog Catalytic Reaction Meaning Learn about homogeneous and heterogeneous. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about the different types. Catalytic Reaction Meaning.
From www.differencebetween.com
Difference Between Catalytic and Non Catalytic Reaction Compare the Catalytic Reaction Meaning catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. Learn about homogeneous and heterogeneous. Learn about the characteristics, types,. Catalytic Reaction Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Reaction Meaning catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. a catalyst is a substance that alters the rate of a reaction without being consumed. a catalyst is a substance that speeds up a chemical reaction without being used up. Learn about homogeneous and heterogeneous. Learn about the different types of catalysts, how. Catalytic Reaction Meaning.
From fyoppxate.blob.core.windows.net
Catalytic Use Meaning at Norman Adair blog Catalytic Reaction Meaning a catalyst is a substance that speeds up a chemical reaction without being used up. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about different types of catalysts, how they work, and their. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn. Catalytic Reaction Meaning.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalytic Reaction Meaning Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. a catalyst is. Catalytic Reaction Meaning.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalytic Reaction Meaning Learn about different types of catalysts, how they work, and their. a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. a catalyst is a substance that alters the rate of a reaction without being consumed. catalysts reduce the activation energy of reactions and enhance the rate of specific. Catalytic Reaction Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Reaction Meaning a catalyst is a substance that speeds up a chemical reaction without being used up. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn about homogeneous and heterogeneous. Learn about. Catalytic Reaction Meaning.
From www.slideserve.com
PPT Catalytic Reaction PowerPoint Presentation, free Catalytic Reaction Meaning a catalyst is a substance that speeds up a chemical reaction without being used up. Learn about the characteristics, types, and. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about homogeneous and heterogeneous. a catalyst is a substance that alters the rate of a reaction without being consumed. a catalyst is. Catalytic Reaction Meaning.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalytic Reaction Meaning a catalyst is a substance that alters the rate of a reaction without being consumed. Learn about homogeneous and heterogeneous. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn about the different types of catalysts, how they work, and. Catalytic Reaction Meaning.
From guidepartrefractor.z21.web.core.windows.net
Energy Diagram Catalyzed Reaction Catalytic Reaction Meaning Learn about different types of catalysts, how they work, and their. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. catalysts reduce the activation energy of reactions and enhance the. Catalytic Reaction Meaning.
From www.slideserve.com
PPT Catalysis & Catalysts PowerPoint Presentation, free download ID Catalytic Reaction Meaning a catalyst is a substance that alters the rate of a reaction without being consumed. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. Learn about different types of catalysts, how they work, and their. a catalyst is a substance that increases the rate of. Catalytic Reaction Meaning.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalytic Reaction Meaning catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. a catalyst is a substance that alters the rate of a reaction without being consumed. Learn about homogeneous. Catalytic Reaction Meaning.
From pediaa.com
Difference Between Thermal Cracking and Catalytic Cracking Definition Catalytic Reaction Meaning Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about homogeneous and heterogeneous. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about the characteristics, types, and. a catalyst is a substance that speeds up a chemical reaction without being used up. . Catalytic Reaction Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Reaction Meaning a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about the characteristics, types, and. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. Learn about the different types of catalysts, how they work, and some examples. Catalytic Reaction Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Reaction Meaning catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. a catalyst is a substance that speeds up a chemical reaction without being used up. Learn about homogeneous and heterogeneous. Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. catalysis is the modification of the rate of a chemical. Catalytic Reaction Meaning.
From www.thoughtco.com
Catalysis Definition in Chemistry Catalytic Reaction Meaning a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. Learn about different types of catalysts, how they work, and their. a catalyst is a substance that alters the rate of a reaction without being consumed. Learn about the characteristics, types, and. catalysis is the modification of the rate. Catalytic Reaction Meaning.
From slidetodoc.com
ENZYME BIOLOGICAL CATALYST Enzyme As Catalyst All enzymes Catalytic Reaction Meaning Learn how catalysts work, types of catalysts, and examples of catalysis in chemistry. Learn about the characteristics, types, and. Learn about different types of catalysts, how they work, and their. a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. Learn about the different types of catalysts, how they work, and. Catalytic Reaction Meaning.
From www.slideserve.com
PPT Mechanisms of Catalytic Reactions and Characterization of Catalytic Reaction Meaning a catalyst is a substance that affects the rate of a chemical reaction by lowering the activation energy. Learn about homogeneous and heterogeneous. catalysis is the modification of the rate of a chemical reaction by addition of a substance not consumed during the reaction. a catalyst is a substance that alters the rate of a reaction without. Catalytic Reaction Meaning.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalytic Reaction Meaning a catalyst is a substance that speeds up a chemical reaction without being used up. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. Learn about the different types of catalysts, how they work, and some examples of catalysis in chemistry and biology. a catalyst is a substance. Catalytic Reaction Meaning.
From www.britannica.com
Catalysis Chemistry, Classification, & Chemical Reactions Britannica Catalytic Reaction Meaning a catalyst is a substance that speeds up a chemical reaction without being used up. catalysts reduce the activation energy of reactions and enhance the rate of specific reactions. Learn about homogeneous and heterogeneous. a catalyst is a substance that increases the rate of a chemical reaction without being consumed by it. a catalyst is a. Catalytic Reaction Meaning.