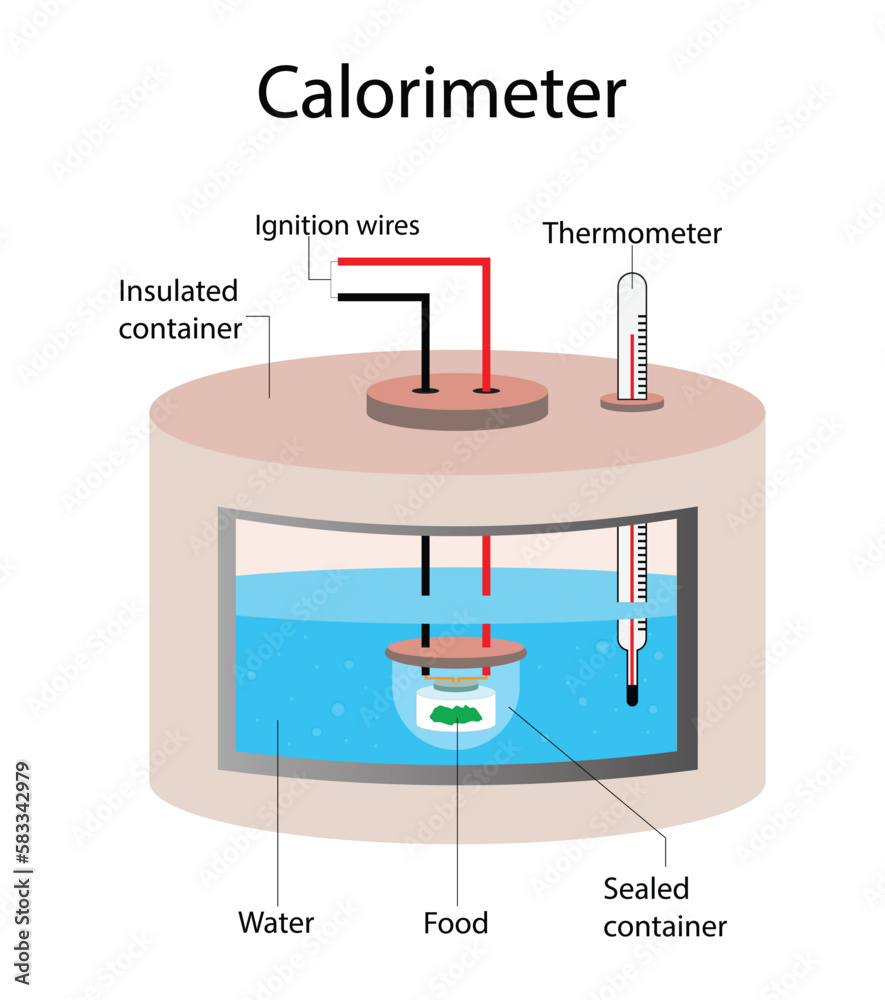
Most Accurate Calorimeter . Calorimetry is a very useful technique to determine. Calorimetry is used to measure amounts of heat transferred to or from a substance. These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: Liquid water has one of the highest specific heats known. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. To do so, the heat is exchanged.
from stock.adobe.com
Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. Calorimetry is a very useful technique to determine. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: Liquid water has one of the highest specific heats known.
Vettoriale Stock illustration of chemistry and physics, Calorimeter
Most Accurate Calorimeter These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. Calorimetry is a very useful technique to determine. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Liquid water has one of the highest specific heats known. To do so, the heat is exchanged. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. Calorimetry is used to measure amounts of heat transferred to or from a substance. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as.
From www.thoughtco.com
Calorimeter Definition in Chemistry Most Accurate Calorimeter To do so, the heat is exchanged. Liquid water has one of the highest specific heats known. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Calorimetry is used to. Most Accurate Calorimeter.
From www.researchgate.net
Schematic representation of calorimeter system US4 at SKINR. Download Most Accurate Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. Liquid water has one of the highest specific heats known. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Calorimetry is a very useful technique to determine. The problem is illustrated. Most Accurate Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Most Accurate Calorimeter Calorimetry is a very useful technique to determine. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. The problem is illustrated by questions frequently. Most Accurate Calorimeter.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Most Accurate Calorimeter To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. Calorimetry is a very useful technique to determine. Liquid water. Most Accurate Calorimeter.
From www.indiamart.com
Digital Bomb Calorimeter Model CC01/M2, डिजिटल बम कैलोरीमीटर Most Accurate Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. Calorimetry is a very useful technique to determine. The problem is illustrated by questions frequently asked by. Most Accurate Calorimeter.
From quizlet.com
Sketch a simple calorimeter and label the purpose of each co Quizlet Most Accurate Calorimeter These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. To do so, the heat is exchanged. Liquid water has one of the highest specific heats known. To determine the enthalpy change of combustion, we must convert joules into the unit. Most Accurate Calorimeter.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Most Accurate Calorimeter The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better. Most Accurate Calorimeter.
From www.researchgate.net
Scheme of the isoperibolic calorimeter. Download Scientific Diagram Most Accurate Calorimeter To do so, the heat is exchanged. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction. Most Accurate Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Most Accurate Calorimeter The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: Calorimetry is a very useful technique to determine. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Calorimetry is used to measure quantities of heat, and can be used to. Most Accurate Calorimeter.
From www.chegg.com
Solved Thermometer A bomb calorimeter, or constant volume Most Accurate Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring. Most Accurate Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Most Accurate Calorimeter These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. Calorimetry is a very useful technique to determine. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: The amount of heat absorbed by the calorimeter. Most Accurate Calorimeter.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams Most Accurate Calorimeter Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. To do so, the. Most Accurate Calorimeter.
From www.researchgate.net
Technological description of the calorimeter prototype Download Most Accurate Calorimeter Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. To do so, the heat is exchanged. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate. Most Accurate Calorimeter.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Most Accurate Calorimeter Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a very useful technique to determine. To do so, the heat is exchanged. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. These calorimeters can be. Most Accurate Calorimeter.
From www.animalia-life.club
Calorimeter Diagram Most Accurate Calorimeter Liquid water has one of the highest specific heats known. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. To do so, the heat is exchanged. The problem. Most Accurate Calorimeter.
From www.semanticscholar.org
Figure 1 from An accurate calorimeterbased method for the thermal Most Accurate Calorimeter Calorimetry is a very useful technique to determine. Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. To do so, the heat is exchanged. To determine the enthalpy change of combustion, we must convert joules into the unit. Most Accurate Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Most Accurate Calorimeter The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. Calorimetry is a very useful technique to determine. To do so, the heat is exchanged. These. Most Accurate Calorimeter.
From dokumen.tips
(PPT) Calorimetry and Heats of Reaction Very often, the most accurate Most Accurate Calorimeter Calorimetry is a very useful technique to determine. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. To do so, the heat is exchanged. These calorimeters can be used to measure both the exothermic. Most Accurate Calorimeter.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Most Accurate Calorimeter Calorimetry is used to measure amounts of heat transferred to or from a substance. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture. Most Accurate Calorimeter.
From saylordotorg.github.io
Calorimetry Most Accurate Calorimeter To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. Liquid water has one of the highest specific heats known. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. To do so, the heat is. Most Accurate Calorimeter.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Most Accurate Calorimeter To do so, the heat is exchanged. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Calorimetry is a very useful technique to determine. Calorimetry is used to measure amounts of heat transferred to or from a substance. These calorimeters can be used to measure. Most Accurate Calorimeter.
From www.researchgate.net
A schematic of the calorimeterbased method for the thermal Most Accurate Calorimeter Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build. Most Accurate Calorimeter.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Most Accurate Calorimeter Calorimetry is a very useful technique to determine. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. Liquid water has one of the highest specific heats known. The amount of heat absorbed by. Most Accurate Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Most Accurate Calorimeter To do so, the heat is exchanged. Calorimetry is used to measure amounts of heat transferred to or from a substance. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp:. Most Accurate Calorimeter.
From www.youtube.com
050 Calorimetry YouTube Most Accurate Calorimeter The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a very useful. Most Accurate Calorimeter.
From www.semanticscholar.org
Figure 1 from An accurate calorimeterbased method for the thermal Most Accurate Calorimeter To do so, the heat is exchanged. The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. Liquid water has one of the highest specific heats known. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: To determine the enthalpy. Most Accurate Calorimeter.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific Most Accurate Calorimeter To do so, the heat is exchanged. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. To determine the enthalpy change of combustion,. Most Accurate Calorimeter.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Most Accurate Calorimeter The amount of heat absorbed by the calorimeter is often small enough that we can neglect it (though not for highly accurate measurements, as. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. Calorimetry. Most Accurate Calorimeter.
From www.walmart.com
Electric Calorimeter Most Accurate Calorimeter The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: Liquid water has one of the highest specific heats known. To do so, the heat is exchanged. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. These calorimeters can be used to measure. Most Accurate Calorimeter.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Most Accurate Calorimeter Calorimetry is a very useful technique to determine. Liquid water has one of the highest specific heats known. These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. The amount of heat absorbed by the calorimeter is often small enough that. Most Accurate Calorimeter.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Most Accurate Calorimeter These calorimeters can be used to measure both the exothermic and endothermic changes within a reaction system to build a better picture of what is occurring during the reaction. Calorimetry is a very useful technique to determine. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure quantities of heat, and. Most Accurate Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry I Most Accurate Calorimeter To do so, the heat is exchanged. Liquid water has one of the highest specific heats known. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. The problem. Most Accurate Calorimeter.
From www.animalia-life.club
Calorimeter Diagram Most Accurate Calorimeter Calorimetry is a very useful technique to determine. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. To do so, the heat is exchanged. The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: To determine the enthalpy change of combustion, we must. Most Accurate Calorimeter.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Most Accurate Calorimeter To do so, the heat is exchanged. To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction. Most Accurate Calorimeter.
From www.researchgate.net
Crosssection of the calorimeter. Download Scientific Diagram Most Accurate Calorimeter The problem is illustrated by questions frequently asked by potential customers of calorimetry sciences corp: To determine the enthalpy change of combustion, we must convert joules into the unit of enthalpy change, joules per mole. Calorimetry is used to measure amounts of heat transferred to or from a substance. These calorimeters can be used to measure both the exothermic and. Most Accurate Calorimeter.