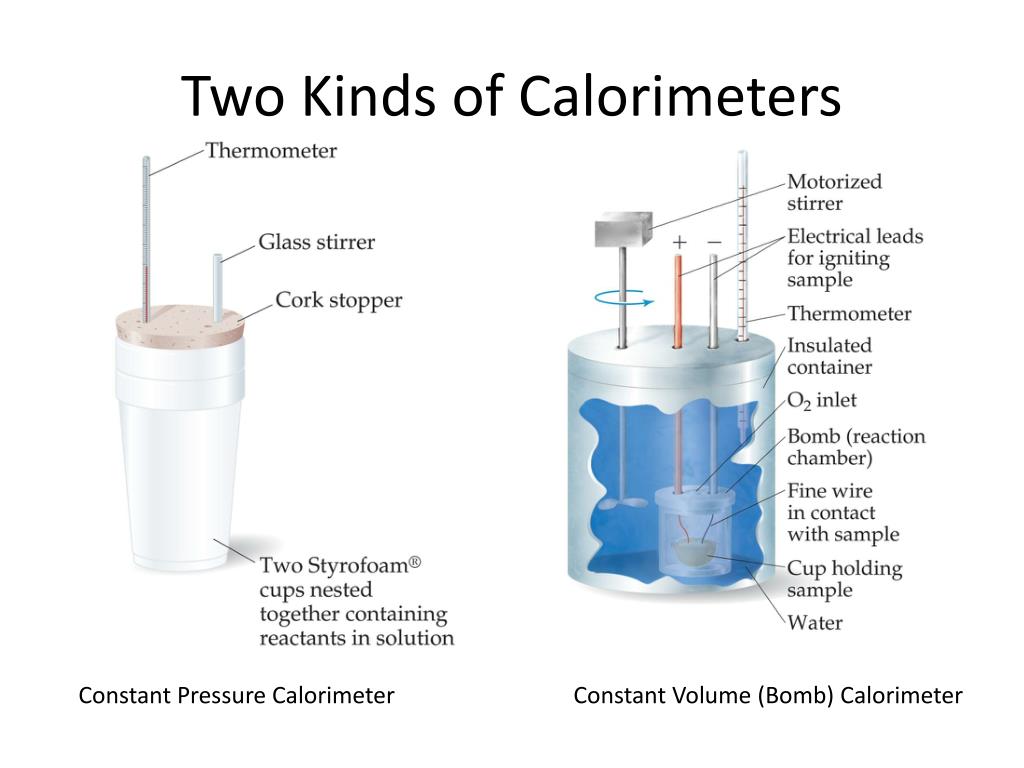
Bbc Calorimetry . Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Enzymes are biological catalysts which speed up reactions. They are specific for their substrate. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Calorimetry is the measurement enthalpy changes in chemical reactions; Place the immersion heater into the central hole at the top of the calorimeter. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. Place one litre (1 kg) of water in the calorimeter. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in.
from www.slideserve.com
A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Place one litre (1 kg) of water in the calorimeter. They are specific for their substrate. Place the immersion heater into the central hole at the top of the calorimeter. Enzymes are biological catalysts which speed up reactions. Calorimetry is the measurement enthalpy changes in chemical reactions; Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to.
PPT Calorimetry PowerPoint Presentation, free download ID3850751
Bbc Calorimetry Calorimetry is the measurement enthalpy changes in chemical reactions; Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Enzymes are biological catalysts which speed up reactions. Place the immersion heater into the central hole at the top of the calorimeter. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. They are specific for their substrate. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Calorimetry is the measurement enthalpy changes in chemical reactions; Place one litre (1 kg) of water in the calorimeter.
From www.researchgate.net
Differential Scanning Calorimetry spectrum of Fe83 Ga17 alloy (a) and Bbc Calorimetry Enzymes are biological catalysts which speed up reactions. Calorimetry is the measurement enthalpy changes in chemical reactions; They are specific for their substrate. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum. Bbc Calorimetry.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness Bbc Calorimetry Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Calorimetry. Bbc Calorimetry.
From glossary.periodni.com
Download calorimeter.png image from Bbc Calorimetry Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. Calorimetry is the measurement enthalpy changes in chemical reactions; Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Enzymes are biological catalysts which speed up reactions. Place the immersion heater into the. Bbc Calorimetry.
From www.semanticscholar.org
Figure 8 from Validation of a new “3D calorimetry” of hot nuclei with Bbc Calorimetry Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance. Bbc Calorimetry.
From www.researchgate.net
a Differential scanning calorimetry and b thermogravimetric analysis Bbc Calorimetry The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Place one litre (1 kg) of water in the calorimeter. Place the immersion heater into the central hole. Bbc Calorimetry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bbc Calorimetry Place the immersion heater into the central hole at the top of the calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Calorimetry is the measurement enthalpy changes in chemical reactions; The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of. Bbc Calorimetry.
From www.chegg.com
Solved 1045 Worksheets FIUBBC Calorimetry Learning Bbc Calorimetry Place the immersion heater into the central hole at the top of the calorimeter. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Enzymes are biological catalysts which. Bbc Calorimetry.
From www.semanticscholar.org
Figure 1 from Validation of a new “3D calorimetry” of hot nuclei with Bbc Calorimetry Place one litre (1 kg) of water in the calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. They are specific for their substrate. Calorimetry is the measurement enthalpy changes in chemical reactions; The enthalpy of combustion of a substance is defined as the heat energy given out when one. Bbc Calorimetry.
From www.labster.com
Calorimetry Using a bomb calorimeter Virtual Lab Bbc Calorimetry Place the immersion heater into the central hole at the top of the calorimeter. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Exothermic reactions in solution. Bbc Calorimetry.
From www.studypool.com
SOLUTION Calorimetry formula sheet Studypool Bbc Calorimetry Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. Place one litre (1 kg) of water in the calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. The enthalpy of combustion of a substance is defined as the heat energy given out when. Bbc Calorimetry.
From studylib.net
Calorimetry Bbc Calorimetry A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Place the immersion heater into the central hole at the top of the calorimeter. Enzymes are biological catalysts which speed up reactions. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance. Bbc Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Bbc Calorimetry Place the immersion heater into the central hole at the top of the calorimeter. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Place one litre (1 kg) of water in the calorimeter. Exothermic reactions in solution give out energy and the temperature increases, while. Bbc Calorimetry.
From www.mdpi.com
Sensors Free FullText Indirect Calorimetry in Spontaneously Bbc Calorimetry They are specific for their substrate. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Place one litre (1 kg) of water in the calorimeter. Place the immersion. Bbc Calorimetry.
From www.semanticscholar.org
Figure 15 from Validation of a new “3D calorimetry” of hot nuclei with Bbc Calorimetry Place one litre (1 kg) of water in the calorimeter. Enzymes are biological catalysts which speed up reactions. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Exothermic reactions in. Bbc Calorimetry.
From www.semanticscholar.org
Figure 1 from Validation of a new “3D calorimetry” of hot nuclei with Bbc Calorimetry A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Calorimetry is the measurement enthalpy changes in chemical reactions; Enzymes are biological catalysts which speed up reactions. They are specific for their substrate. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. Calorimetry is used to. Bbc Calorimetry.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Bbc Calorimetry Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Place the immersion heater into the central hole at the top of the calorimeter. They. Bbc Calorimetry.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Bbc Calorimetry The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Place one litre (1 kg) of water in the calorimeter. Calorimetry is the measurement enthalpy changes in chemical reactions; A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can;. Bbc Calorimetry.
From www.semanticscholar.org
Figure 7 from Validation of a new “3D calorimetry” of hot nuclei with Bbc Calorimetry Place the immersion heater into the central hole at the top of the calorimeter. Calorimetry is the measurement enthalpy changes in chemical reactions; Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. They are specific. Bbc Calorimetry.
From www.researchgate.net
A) Differential scanning calorimetry thermograms of the crystalline Bbc Calorimetry The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. They are specific for their substrate. Calorimetry is the measurement enthalpy changes in chemical reactions; Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments.. Bbc Calorimetry.
From chemistrytalk.org
Calorimetry ChemTalk Bbc Calorimetry A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Place one litre (1 kg) of water in the calorimeter. They are specific for their substrate. Calorimetry is the measurement enthalpy changes in chemical reactions; Place the immersion heater into the central hole at the top of the calorimeter. Enzymes are biological catalysts. Bbc Calorimetry.
From www.docsity.com
Calorimetry and Hess's Law in Chemistry Lab Experiment 3 CHEM 1 Bbc Calorimetry The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Calorimetry is the measurement enthalpy changes in chemical reactions; Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Place the immersion heater into the. Bbc Calorimetry.
From www.chegg.com
Solved 1045 Worksheets FIUBBC Calorimetry Reactions and Bbc Calorimetry Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Calorimetry. Bbc Calorimetry.
From www.semanticscholar.org
Figure 12 from Validation of a new “3D calorimetry” of hot nuclei with Bbc Calorimetry They are specific for their substrate. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is the measurement enthalpy changes in chemical reactions; Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. Place the immersion heater into the central hole. Bbc Calorimetry.
From www.semanticscholar.org
Figure 6 from Validation of a new “3D calorimetry” of hot nuclei with Bbc Calorimetry The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Place one litre (1 kg) of water in the calorimeter. They are specific for their substrate. Exothermic reactions in solution give out energy and the temperature increases, while endothermic reactions take in. Enzymes are biological catalysts. Bbc Calorimetry.
From www.science-revision.co.uk
Calorimetry Bbc Calorimetry A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Place the immersion heater into the central hole at the top of the calorimeter. They are specific for their substrate. Calorimetry is the measurement enthalpy changes in chemical reactions; Calorimetry is used to measure quantities of heat, and can be used to determine. Bbc Calorimetry.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Bbc Calorimetry Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Enzymes are biological catalysts which speed up reactions. Place the immersion heater into the central. Bbc Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry I Bbc Calorimetry Place the immersion heater into the central hole at the top of the calorimeter. Calorimetry is the measurement enthalpy changes in chemical reactions; They are specific for their substrate. Enzymes are biological catalysts which speed up reactions. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Place one. Bbc Calorimetry.
From www.chegg.com
Solved Physics 111 Lab 202 Calorimetry Introduction A Bbc Calorimetry Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; The enthalpy of combustion. Bbc Calorimetry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Bbc Calorimetry Place one litre (1 kg) of water in the calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Place the immersion heater into the central hole at the top of the calorimeter. Calorimetry is the measurement enthalpy changes in chemical reactions; A simple calorimeter can be made from a polystyrene. Bbc Calorimetry.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.3 Calorimetry翰林国际教育 Bbc Calorimetry They are specific for their substrate. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Calorimetry is the measurement enthalpy changes in chemical reactions; A simple calorimeter can be made from a polystyrene drinking cup, a vacuum flask or metal can; Place the immersion heater. Bbc Calorimetry.
From laboratorytests.org
Colorimeter Principle, Instrumentation and Uses Bbc Calorimetry Enzymes are biological catalysts which speed up reactions. The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Place the immersion heater into the central hole at the top of the calorimeter. Place one litre (1 kg) of water in the calorimeter. Exothermic reactions in solution. Bbc Calorimetry.
From www.chegg.com
Solved PART II CALORIMETRY (Record data from the Energy Bbc Calorimetry Enzymes are biological catalysts which speed up reactions. Place the immersion heater into the central hole at the top of the calorimeter. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features. Bbc Calorimetry.
From www.tffn.net
Solving Calorimetry Problems A StepbyStep Guide The Enlightened Bbc Calorimetry Calorimetry is the measurement enthalpy changes in chemical reactions; Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. They are specific for their substrate. Place the immersion heater into the central hole at the top of the calorimeter. Enzymes are biological catalysts which speed up reactions. Exothermic reactions in solution give. Bbc Calorimetry.
From wechat.engineering.org.cn
New Developments in the Calorimetry of HighTemperature Materials Bbc Calorimetry The enthalpy of combustion of a substance is defined as the heat energy given out when one mole of a substance burns completely in. Place one litre (1 kg) of water in the calorimeter. Calorimetry is the measurement enthalpy changes in chemical reactions; Enzymes are biological catalysts which speed up reactions. Place the immersion heater into the central hole at. Bbc Calorimetry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bbc Calorimetry Place one litre (1 kg) of water in the calorimeter. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through experiments. They are specific for their substrate. A simple calorimeter can be. Bbc Calorimetry.