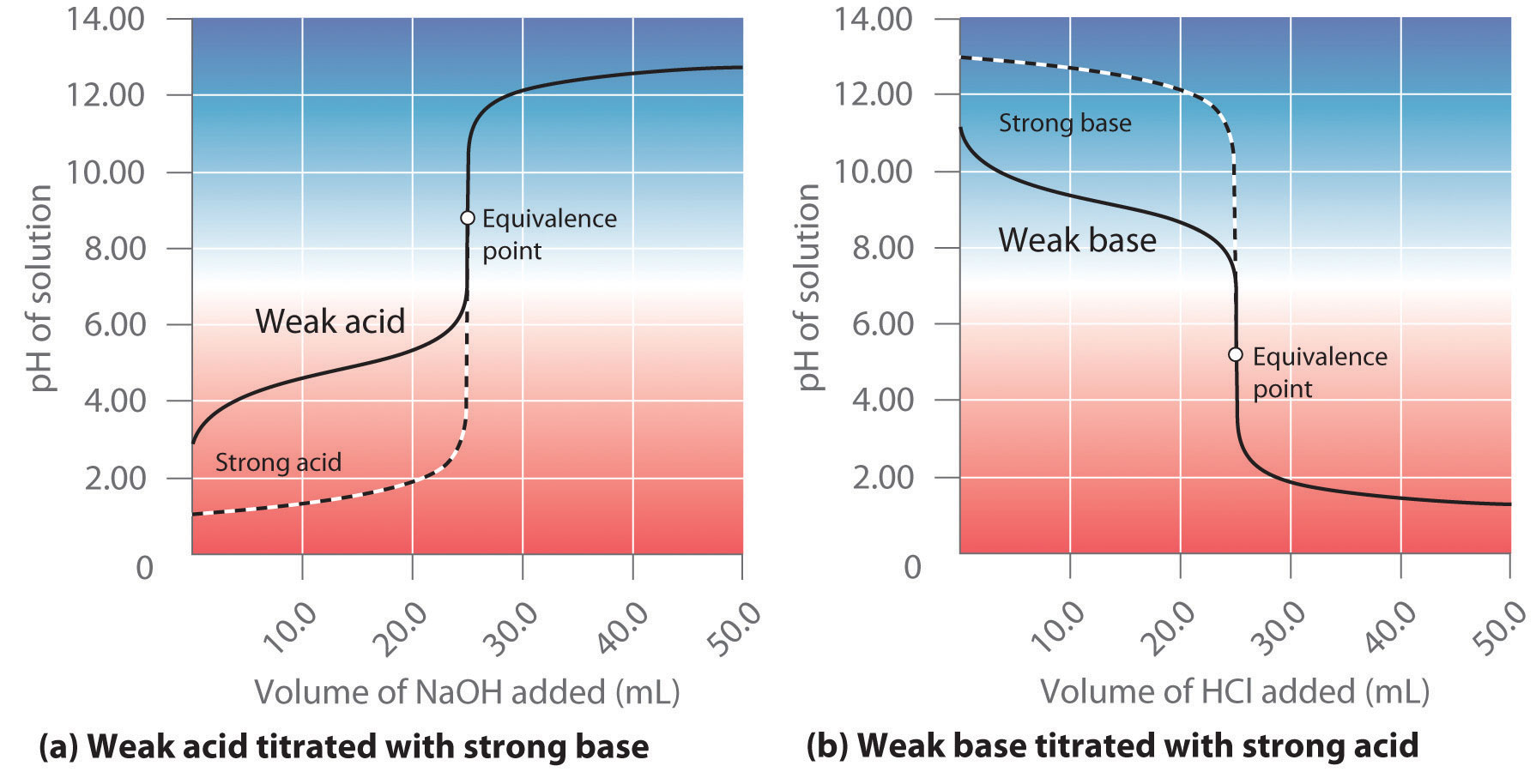
Titration Curve Indicator . A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a plot of some solution property versus the amount of added titrant. The diagram shows the ph curve between strong acid to a. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. The figure below shows two different examples of a strong. This page assumes that you know. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. To choose an indicator it varies from titration to titration. The end point of a titration is the point at which an indicator changes. Strong acid v strong base.
from chem.libretexts.org
Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of a strong. The end point of a titration is the point at which an indicator changes. To choose an indicator it varies from titration to titration. A titration curve is a plot of some solution property versus the amount of added titrant. Strong acid v strong base. This page assumes that you know. The diagram shows the ph curve between strong acid to a. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. A titration curve is a graphical representation of the ph of a solution during a titration.
15.6 AcidBase Titration Curves Chemistry LibreTexts
Titration Curve Indicator The figure below shows two different examples of a strong. A titration curve is a plot of some solution property versus the amount of added titrant. The figure below shows two different examples of a strong. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. The end point of a titration is the point at which an indicator changes. Strong acid v strong base. The diagram shows the ph curve between strong acid to a. This page assumes that you know. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. To choose an indicator it varies from titration to titration. A titration curve is a graphical representation of the ph of a solution during a titration.
From wisc.pb.unizin.org
D30.1 AcidBase Indicators Chemistry 109 Fall 2021 Titration Curve Indicator A titration curve is a graphical representation of the ph of a solution during a titration. The diagram shows the ph curve between strong acid to a. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. Strong acid. Titration Curve Indicator.
From ar.inspiredpencil.com
Titration Diagram Titration Curve Indicator Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. To choose an indicator it varies from titration to titration. The end point of a titration is the point at which an indicator changes. Strong acid v strong base.. Titration Curve Indicator.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Curve Indicator A titration curve is a graphical representation of the ph of a solution during a titration. To choose an indicator it varies from titration to titration. Strong acid v strong base. This page assumes that you know. The diagram shows the ph curve between strong acid to a. Titration curves show how the ph of an acidic or basic solution. Titration Curve Indicator.
From www.youtube.com
how to draw AcidBase titration Curve and selection of Suitable Titration Curve Indicator Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a plot. Titration Curve Indicator.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Indicator To choose an indicator it varies from titration to titration. A titration curve is a graphical representation of the ph of a solution during a titration. The diagram shows the ph curve between strong acid to a. This page assumes that you know. Titration curves show how the ph of an acidic or basic solution changes as a basic or. Titration Curve Indicator.
From www.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Chemistry JoVE Titration Curve Indicator The end point of a titration is the point at which an indicator changes. A titration curve is a plot of some solution property versus the amount of added titrant. Strong acid v strong base. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence. Titration Curve Indicator.
From mungfali.com
Acid Base Titration Indicator Titration Curve Indicator A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. A titration curve is a plot of some solution property versus the amount of added titrant. The figure below shows two different examples of a strong. The diagram shows the. Titration Curve Indicator.
From www.numerade.com
SOLVED Question 4 (1 point) The following graph is a titration curve Titration Curve Indicator The figure below shows two different examples of a strong. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. To choose an indicator it varies from titration to titration. A titration curve is a plot of some solution property versus the amount of added titrant. This. Titration Curve Indicator.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Titration Curve Indicator A titration curve is a graphical representation of the ph of a solution during a titration. The diagram shows the ph curve between strong acid to a. A titration curve is a plot of some solution property versus the amount of added titrant. The end point of a titration is the point at which an indicator changes. Strong acid v. Titration Curve Indicator.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Indicator Strong acid v strong base. The diagram shows the ph curve between strong acid to a. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a plot of some solution property versus the amount of. Titration Curve Indicator.
From www.youtube.com
Titration curves and acidbase indicators Chemistry Khan Academy Titration Curve Indicator This page assumes that you know. The end point of a titration is the point at which an indicator changes. The diagram shows the ph curve between strong acid to a. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show. Titration Curve Indicator.
From www.numerade.com
SOLVEDSuggest an appropriate Indicator for each equivalence point Titration Curve Indicator The figure below shows two different examples of a strong. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. A titration curve is a plot of some solution property versus the amount of added titrant. Strong acid v. Titration Curve Indicator.
From www.vedantu.com
Redox Titration Permanganate, Dichromate and Idometric Titration Titration Curve Indicator To choose an indicator it varies from titration to titration. The diagram shows the ph curve between strong acid to a. The end point of a titration is the point at which an indicator changes. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a. Titration Curve Indicator.
From www.chegg.com
Solved Titration Curve Using the table of indicators Titration Curve Indicator Strong acid v strong base. To choose an indicator it varies from titration to titration. A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a plot of some solution property versus the amount of added titrant. The figure below shows two different examples of a strong. The end point. Titration Curve Indicator.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Indicator The diagram shows the ph curve between strong acid to a. To choose an indicator it varies from titration to titration. Strong acid v strong base. The figure below shows two different examples of a strong. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the. Titration Curve Indicator.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Indicator Strong acid v strong base. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. The end point of a titration is the point at which an indicator changes. To choose an indicator it varies from titration to titration.. Titration Curve Indicator.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Indicator Strong acid v strong base. The diagram shows the ph curve between strong acid to a. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of. Titration Curve Indicator.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Indicator Strong acid v strong base. A titration curve is a plot of some solution property versus the amount of added titrant. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. This page assumes that you know. The end. Titration Curve Indicator.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Titration Curve Indicator A titration curve is a plot of some solution property versus the amount of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at which an indicator changes. The diagram shows the ph curve between strong acid to a. The figure below. Titration Curve Indicator.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Titration Curve Indicator This page assumes that you know. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Strong acid v strong base. A titration curve is a graphical representation of the ph of a solution during a titration. To choose an indicator it varies from titration to titration.. Titration Curve Indicator.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curve Indicator The diagram shows the ph curve between strong acid to a. Strong acid v strong base. A titration curve is a plot of some solution property versus the amount of added titrant. The figure below shows two different examples of a strong. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic. Titration Curve Indicator.
From www.studypool.com
SOLUTION Acid base indicators titration curves Studypool Titration Curve Indicator Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The diagram shows the ph curve between strong acid to a. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point. Titration Curve Indicator.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Indicator The end point of a titration is the point at which an indicator changes. The diagram shows the ph curve between strong acid to a. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. This page assumes that you know. To choose an indicator it varies. Titration Curve Indicator.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Indicator The diagram shows the ph curve between strong acid to a. A titration curve is a plot of some solution property versus the amount of added titrant. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. To choose. Titration Curve Indicator.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Titration Curve Indicator The figure below shows two different examples of a strong. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. Strong acid v strong base. To choose an indicator it varies from titration to titration. The end point of a titration is the point at which an. Titration Curve Indicator.
From www.linstitute.net
CIE A Level Chemistry复习笔记1.7.12 pH Titration Curves翰林国际教育 Titration Curve Indicator The figure below shows two different examples of a strong. A titration curve is a plot of some solution property versus the amount of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic. Titration Curve Indicator.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Titration Curve Indicator The end point of a titration is the point at which an indicator changes. A titration curve is a plot of some solution property versus the amount of added titrant. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation. Titration Curve Indicator.
From mmerevise.co.uk
pH Curves Questions and Revision MME Titration Curve Indicator The figure below shows two different examples of a strong. To choose an indicator it varies from titration to titration. This page assumes that you know. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. A titration curve is a graphical representation of the ph of. Titration Curve Indicator.
From slideplayer.com
Acid Base Titration Curves & Indicators ppt download Titration Curve Indicator Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. A titration curve is a plot of some solution property versus the amount of added titrant. The diagram shows the ph curve between strong acid to a. A titration. Titration Curve Indicator.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Indicator Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. The end point of a titration is the point at which an indicator changes. The figure below shows two different examples of a strong. This page assumes that you. Titration Curve Indicator.
From www.numerade.com
SOLVED Using the table of indicators identify which of the given Titration Curve Indicator The diagram shows the ph curve between strong acid to a. Due to the steepness of the titration curve of a strong acid around the equivalence point, either indicator will rapidly change color at the equivalence point for the titration of the. This page assumes that you know. Titration curves show how the ph of an acidic or basic solution. Titration Curve Indicator.
From mungfali.com
Titration Graph Titration Curve Indicator Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a titration. Due to the steepness of the titration curve of a strong. Titration Curve Indicator.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curve Indicator This page assumes that you know. To choose an indicator it varies from titration to titration. The diagram shows the ph curve between strong acid to a. Strong acid v strong base. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. The end. Titration Curve Indicator.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Titration Curve Indicator A titration curve is a graphical representation of the ph of a solution during a titration. To choose an indicator it varies from titration to titration. The end point of a titration is the point at which an indicator changes. The diagram shows the ph curve between strong acid to a. Due to the steepness of the titration curve of. Titration Curve Indicator.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Curve Indicator A titration curve is a graphical representation of the ph of a solution during a titration. This page assumes that you know. The end point of a titration is the point at which an indicator changes. Titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The. Titration Curve Indicator.