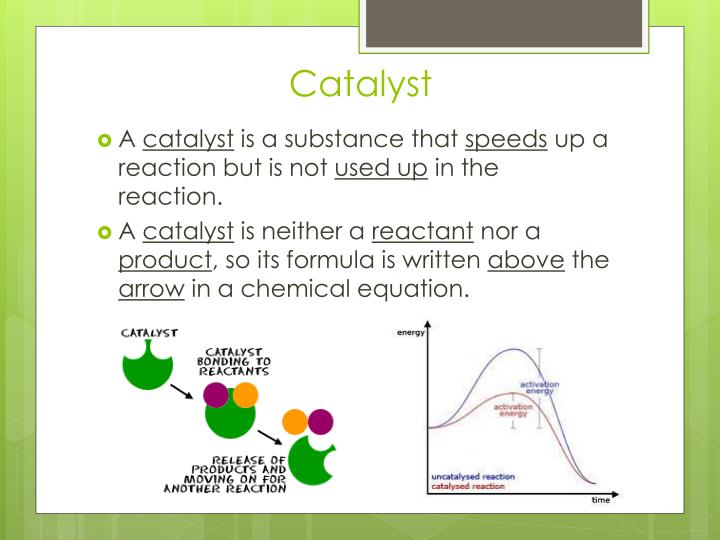
Are Catalysts In Equation . The mass of a catalyst at the beginning and end of a reaction is the same. Catalysts do not appear in the overall chemical equation for a reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Because it is neither a reactant nor a. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. Catalysts play an important part in many chemical processes. A catalyst provides an alternative. So catalysts can be used over and over again. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. After the reaction occurs, a catalyst returns to its original state;
from www.slideserve.com
Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts do not appear in the overall chemical equation for a reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. Normally only small amounts of catalysts are needed to have an effect on a reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts participate in a chemical reaction and increase its rate. After the reaction occurs, a catalyst returns to its original state; Catalysts play an important part in many chemical processes.
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID6634764
Are Catalysts In Equation The mass of a catalyst at the beginning and end of a reaction is the same. A catalyst provides an alternative. So catalysts can be used over and over again. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. Catalysts participate in a chemical reaction and increase its rate. The mass of a catalyst at the beginning and end of a reaction is the same. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts do not appear in the overall chemical equation for a reaction. Because it is neither a reactant nor a. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts play an important part in many chemical processes. After the reaction occurs, a catalyst returns to its original state; Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.
From www.youtube.com
Practice Identifying Catalysts and Intermediates YouTube Are Catalysts In Equation Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Normally only small amounts of catalysts are needed to have an effect on a reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. In general, catalytic. Are Catalysts In Equation.
From www.researchgate.net
Scheme 3. Chemical equation for the catalytic cleavage of the βO4 Are Catalysts In Equation Catalysts do not appear in the overall chemical equation for a reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. So catalysts can be used over and over again. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalyst, in chemistry, any substance that increases the rate. Are Catalysts In Equation.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Are Catalysts In Equation Because it is neither a reactant nor a. Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. So catalysts can be used over and over again. Catalysts participate in a chemical reaction and increase its. Are Catalysts In Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation ID6634764 Are Catalysts In Equation After the reaction occurs, a catalyst returns to its original state; Catalysts participate in a chemical reaction and increase its rate. Catalysts do not appear in the overall chemical equation for a reaction. Catalysts play an important part in many chemical processes. The mass of a catalyst at the beginning and end of a reaction is the same. In general,. Are Catalysts In Equation.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Are Catalysts In Equation Catalysts do not appear in the overall chemical equation for a reaction. Catalysts participate in a chemical reaction and increase its rate. After the reaction occurs, a catalyst returns to its original state; So catalysts can be used over and over again. The mass of a catalyst at the beginning and end of a reaction is the same. They do. Are Catalysts In Equation.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Are Catalysts In Equation Catalysts participate in a chemical reaction and increase its rate. The mass of a catalyst at the beginning and end of a reaction is the same. So catalysts can be used over and over again. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. After the reaction occurs, a catalyst returns to its original state; Because it is. Are Catalysts In Equation.
From www.slideserve.com
PPT Starter 1)Definition of catalysts 2) Difference between Are Catalysts In Equation Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts play an important part in many chemical processes. Catalysts participate in a chemical reaction and increase its rate. The mass of a catalyst at the beginning and end of a reaction is the same. Catalyst, in chemistry, any substance that. Are Catalysts In Equation.
From www.sciencelearn.org.nz
Catalytic converter catalyst — Science Learning Hub Are Catalysts In Equation Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. They do not appear in the reaction’s net equation and are not consumed during the reaction. A catalyst provides an alternative. Catalysts do not appear in the overall chemical equation for a reaction. So catalysts can be used over and. Are Catalysts In Equation.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub Are Catalysts In Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalysts do not appear in the overall chemical equation for a reaction. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed. Are Catalysts In Equation.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Are Catalysts In Equation In general, catalytic action is a chemical reaction between the catalyst and a reactant. They do not appear in the reaction’s net equation and are not consumed during the reaction. So catalysts can be used over and over again. Catalysts participate in a chemical reaction and increase its rate. The mass of a catalyst at the beginning and end of. Are Catalysts In Equation.
From www.alamy.com
Chemical Reactions of catalyst and product Stock Vector Image & Art Alamy Are Catalysts In Equation Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Because it is neither a reactant nor a. They do not appear in the reaction’s net equation and are not consumed during the reaction. So catalysts can be used over and over again. Catalysts do not appear in the overall chemical. Are Catalysts In Equation.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Are Catalysts In Equation Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. So catalysts can be used over and over again. Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalysts do not appear in the overall chemical equation for a reaction. They do not appear in the reaction’s net. Are Catalysts In Equation.
From ar.inspiredpencil.com
Catalyst Equation Are Catalysts In Equation A catalyst provides an alternative. So catalysts can be used over and over again. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. After the reaction occurs, a catalyst returns to its original state; Catalysts participate in a chemical reaction and increase its rate. The mass of a catalyst at the beginning and. Are Catalysts In Equation.
From www.slideserve.com
PPT Heterogeneous Catalysis & Solid State Physics PowerPoint Are Catalysts In Equation Catalysts play an important part in many chemical processes. After the reaction occurs, a catalyst returns to its original state; Because it is neither a reactant nor a. Catalysts do not appear in the overall chemical equation for a reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. So catalysts can be used over and over again.. Are Catalysts In Equation.
From present5.com
Brief History of the Catalytic Converter The catalytic Are Catalysts In Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts play an important part in many chemical processes. After the reaction occurs, a catalyst returns to its original state; Normally only small amounts of catalysts are needed. Are Catalysts In Equation.
From slideplayer.com
and Chemical Equilibrium ppt download Are Catalysts In Equation Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalysts play an important part in many chemical processes. In general, catalytic action is a chemical reaction between the catalyst and a reactant. A catalyst provides an alternative. So catalysts can be used over and over again. Catalysts are substances which speed up the rate. Are Catalysts In Equation.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Are Catalysts In Equation In general, catalytic action is a chemical reaction between the catalyst and a reactant. Because it is neither a reactant nor a. Catalysts play an important part in many chemical processes. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts participate in a chemical reaction and increase its rate. Catalysts allow a. Are Catalysts In Equation.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Are Catalysts In Equation So catalysts can be used over and over again. Catalysts do not appear in the overall chemical equation for a reaction. Because it is neither a reactant nor a. Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.. Are Catalysts In Equation.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Are Catalysts In Equation The mass of a catalyst at the beginning and end of a reaction is the same. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts do not appear in the overall chemical equation for a reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In general, catalytic action is. Are Catalysts In Equation.
From 2012books.lardbucket.org
Catalysis Are Catalysts In Equation Catalysts do not appear in the overall chemical equation for a reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. So catalysts can be used over and over again. Catalysts participate in a chemical reaction and increase its. Are Catalysts In Equation.
From klaawqorl.blob.core.windows.net
Examples Of Catalyze Chemical Reactions at Rolf Mock blog Are Catalysts In Equation The mass of a catalyst at the beginning and end of a reaction is the same. Catalysts participate in a chemical reaction and increase its rate. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts play an important part in many chemical processes. Normally only small amounts of catalysts are needed to. Are Catalysts In Equation.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Are Catalysts In Equation So catalysts can be used over and over again. Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. After the reaction occurs, a catalyst returns to its original state; Catalysts allow a reaction to proceed. Are Catalysts In Equation.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Are Catalysts In Equation In general, catalytic action is a chemical reaction between the catalyst and a reactant. Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalysts play an important part in many chemical processes. Because it is neither a reactant nor a. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts are substances. Are Catalysts In Equation.
From loemotkul.blob.core.windows.net
What Is A Catalyst In A Chemical Reaction at Richard Starr blog Are Catalysts In Equation After the reaction occurs, a catalyst returns to its original state; Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts do not appear in the overall chemical equation for a reaction. A catalyst provides an alternative. Catalysts play an important part in many chemical processes. They do not appear. Are Catalysts In Equation.
From www.chemistrystudent.com
Homogeneous Catalysis (ALevel) ChemistryStudent Are Catalysts In Equation Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. Catalysts do not appear in the overall chemical equation for a reaction. Catalysts play an important part in many chemical processes. After the reaction occurs, a catalyst returns to its original state; In general, catalytic action is a chemical reaction. Are Catalysts In Equation.
From www.nagwa.com
Question Video Describing the Effect of Catalysts on Reaction Rates Are Catalysts In Equation Catalysts do not appear in the overall chemical equation for a reaction. So catalysts can be used over and over again. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The mass of a catalyst at the beginning and end of a reaction is the same. A catalyst provides an. Are Catalysts In Equation.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube Are Catalysts In Equation Catalysts play an important part in many chemical processes. A catalyst provides an alternative. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Normally only small amounts of catalysts are needed to have an effect on a reaction. The mass of a catalyst at the beginning and end of a reaction is the. Are Catalysts In Equation.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent Are Catalysts In Equation Catalysts play an important part in many chemical processes. After the reaction occurs, a catalyst returns to its original state; A catalyst provides an alternative. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysts are substances which speed up the rate. Are Catalysts In Equation.
From www.slideserve.com
PPT Reaction Mechanism PowerPoint Presentation, free download ID Are Catalysts In Equation Catalysts participate in a chemical reaction and increase its rate. The mass of a catalyst at the beginning and end of a reaction is the same. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed. Are Catalysts In Equation.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Are Catalysts In Equation Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. After the reaction occurs, a catalyst returns to its original state; Catalysts do not appear in the overall. Are Catalysts In Equation.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Are Catalysts In Equation Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. Catalysts participate in a chemical reaction and increase its rate. Because it is neither a reactant nor a. They do not appear in the reaction’s net equation and are not consumed during the reaction. Normally only small amounts of catalysts. Are Catalysts In Equation.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Are Catalysts In Equation They do not appear in the reaction’s net equation and are not consumed during the reaction. Catalysts participate in a chemical reaction and increase its rate. Normally only small amounts of catalysts are needed to have an effect on a reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts. Are Catalysts In Equation.
From www.scribd.com
Types of Catalysts Arrhenius Equation and Reaction Mechanisms Are Catalysts In Equation Because it is neither a reactant nor a. The mass of a catalyst at the beginning and end of a reaction is the same. Catalysts participate in a chemical reaction and increase its rate. Normally only small amounts of catalysts are needed to have an effect on a reaction. So catalysts can be used over and over again. After the. Are Catalysts In Equation.
From www.slideshare.net
Catalysis Are Catalysts In Equation Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts participate in a chemical reaction and increase its rate. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that. Are Catalysts In Equation.
From www.youtube.com
How does a CATALYST work ? YouTube Are Catalysts In Equation Catalysts are substances which speed up the rate of a reaction without themselves being altered or consumed in the reaction. The mass of a catalyst at the beginning and end of a reaction is the same. Normally only small amounts of catalysts are needed to have an effect on a reaction. Catalyst, in chemistry, any substance that increases the rate. Are Catalysts In Equation.