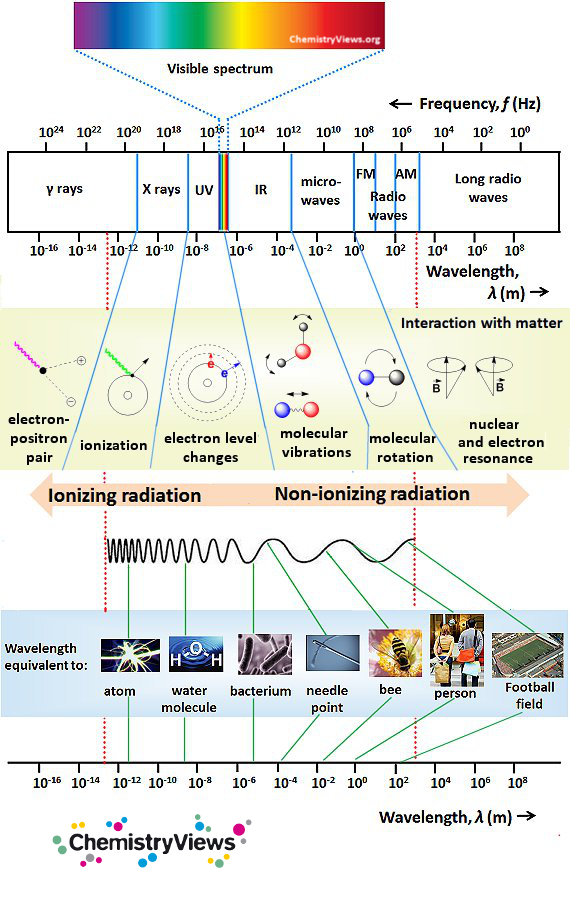
How Do Quantum Jumps Explain Electromagnetic Spectrum . The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. The photons carried away the angular momentum. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. • quantum jumps between energy. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus.
from www.chemistryviews.org
Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. • quantum jumps between energy. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. The photons carried away the angular momentum.
The Spectrum ChemistryViews
How Do Quantum Jumps Explain Electromagnetic Spectrum • quantum jumps between energy. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. • quantum jumps between energy. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. The photons carried away the angular momentum. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones.
From mynewblogsteetburt258.blogspot.com
WHAT IS SPECTRUM ? / EXPLAIN ITS FOLLOWING BANDS How Do Quantum Jumps Explain Electromagnetic Spectrum According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. Quantum jumps are the discrete. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From hubblesite.org
The Spectrum HubbleSite How Do Quantum Jumps Explain Electromagnetic Spectrum According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to classical electromagnetism, the theory. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.slideserve.com
PPT The ELECTRON Wave Particle Duality PowerPoint Presentation How Do Quantum Jumps Explain Electromagnetic Spectrum The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. The photons carried away the angular momentum. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. According to this model, when. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.differencebetween.com
Difference Between Wave Theory and Planck's Quantum How Do Quantum Jumps Explain Electromagnetic Spectrum The photons carried away the angular momentum. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.pinterest.com
Visible Light and the Spectrum How Do Quantum Jumps Explain Electromagnetic Spectrum The photons carried away the angular momentum. • quantum jumps between energy. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. The. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.moomoomathblog.com
What is the SPECTRUM MooMooMath and Science How Do Quantum Jumps Explain Electromagnetic Spectrum The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to classical electromagnetism, the theory that describes how charged particles attract and. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From telegra.ph
Learning the Spectrum Telegraph How Do Quantum Jumps Explain Electromagnetic Spectrum The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. • quantum jumps between energy. The photons carried away the angular momentum. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus.. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From bigthink.com
Quantum jumps How Niels Bohr changed the world Big Think How Do Quantum Jumps Explain Electromagnetic Spectrum According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. • quantum jumps between energy. The photons carried away the angular momentum. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. Quantum jumps are the discrete. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.youtube.com
What is a Quantum Jump Explained in this Diagram YouTube How Do Quantum Jumps Explain Electromagnetic Spectrum According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. Quantum jumps are the discrete. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.youtube.com
Spectrum Basic Introduction YouTube How Do Quantum Jumps Explain Electromagnetic Spectrum The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. The photons carried away the. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From education-portal.com
Electron Transitions & Spectral Lines Video & Lesson Transcript How Do Quantum Jumps Explain Electromagnetic Spectrum According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According to this model, when an electron in a box is subjected. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From byjus.com
Spectrum Definition, Characteristics, Range, Diagram How Do Quantum Jumps Explain Electromagnetic Spectrum The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to this model, when an electron in a box is subjected to. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From discovertutoring.co.uk
Spectrum Explained Discover Tutoring How Do Quantum Jumps Explain Electromagnetic Spectrum According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. The photons carried away the angular momentum. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. The emission spectrum of an element consisted of the photons. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From quizlet.com
Draw and label the spectrum. Diagram Quizlet How Do Quantum Jumps Explain Electromagnetic Spectrum Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. The. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From xn--webducation-dbb.com
What Happens During a Quantum Jump? Education How Do Quantum Jumps Explain Electromagnetic Spectrum The photons carried away the angular momentum. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. • quantum jumps between energy. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From telegra.ph
Understanding the Spectrum Telegraph How Do Quantum Jumps Explain Electromagnetic Spectrum Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. The photons carried away the angular momentum. • quantum jumps between energy. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. The emission spectrum of an element consisted of the photons (or radiation) electrons. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.youtube.com
What is the SPECTRUM YouTube How Do Quantum Jumps Explain Electromagnetic Spectrum Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. The. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From telegra.ph
An understanding of the Spectrum Telegraph How Do Quantum Jumps Explain Electromagnetic Spectrum • quantum jumps between energy. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. Explores why schrödinger proposed quantum beats in. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From mungfali.com
Spectrum C70 How Do Quantum Jumps Explain Electromagnetic Spectrum Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. The photons carried away the angular momentum. The emission spectrum of an element. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From telegra.ph
Understanding how to understand the Spectrum Telegraph How Do Quantum Jumps Explain Electromagnetic Spectrum According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. Explores why schrödinger proposed quantum. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.electricity-magnetism.org
How are waves used in quantum communication and computing? How Do Quantum Jumps Explain Electromagnetic Spectrum Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. • quantum jumps between energy. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.researchgate.net
Spectroscopy and quantum jumps. (A) Pulsed twotone spectroscopy color How Do Quantum Jumps Explain Electromagnetic Spectrum Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. The. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From bigthink.com
Quantum jumps How Niels Bohr changed the world Big Think How Do Quantum Jumps Explain Electromagnetic Spectrum Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. According to this model, when an electron in a box is subjected to. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.carolina.com
Infographic What Is the Spectrum? How Do Quantum Jumps Explain Electromagnetic Spectrum Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. • quantum jumps between energy. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According to classical electromagnetism, the theory that describes. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.blog.sindibad.tn
Quantum Electrodynamics and Feynman Diagrams Magic of Science How Do Quantum Jumps Explain Electromagnetic Spectrum According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. The photons carried away the angular momentum. Explores why schrödinger proposed quantum. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.pinterest.com
radiation types of radiation Kids Encyclopedia How Do Quantum Jumps Explain Electromagnetic Spectrum Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.researchgate.net
Principle of the quantum jump spectroscopy method. Left Relevant How Do Quantum Jumps Explain Electromagnetic Spectrum • quantum jumps between energy. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones.. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts How Do Quantum Jumps Explain Electromagnetic Spectrum Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. According to classical electromagnetism, the theory. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum How Do Quantum Jumps Explain Electromagnetic Spectrum The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. The photons carried away the angular momentum. • quantum jumps between energy. According to classical electromagnetism, the theory that describes how charged particles attract. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From lakhimi.blogspot.com
PAPER 604 RADIATION (EMR) How Do Quantum Jumps Explain Electromagnetic Spectrum According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is a linear. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. • quantum jumps between energy.. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From kokngoyomen.blogspot.com
Spectrum Visible Light Spectrum Wavelengths / The How Do Quantum Jumps Explain Electromagnetic Spectrum • quantum jumps between energy. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation,. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From byjus.com
What do you mean by spectrum? Give the complete How Do Quantum Jumps Explain Electromagnetic Spectrum Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From fity.club
The Spectrum How Do Quantum Jumps Explain Electromagnetic Spectrum The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to classical electromagnetism, the theory that describes how charged particles attract and. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From www.chemistryviews.org
The Spectrum ChemistryViews How Do Quantum Jumps Explain Electromagnetic Spectrum Explores why schrödinger proposed quantum beats in wavefunctions to explain line spectra. Quantum jumps are the discrete transitions between different energy levels of a quantum system, such as an atom or molecule, typically involving the. According to classical electromagnetism, the theory that describes how charged particles attract and repel one another, an electron would spiral down to the nucleus. •. How Do Quantum Jumps Explain Electromagnetic Spectrum.
From sites.pitt.edu
Origins of Quantum Theory How Do Quantum Jumps Explain Electromagnetic Spectrum The photons carried away the angular momentum. The emission spectrum of an element consisted of the photons (or radiation) electrons gave off when they jumped from higher orbits to lower ones. According to this model, when an electron in a box is subjected to a perturbation such as electromagnetic radiation, the electron responds by going into a state which is. How Do Quantum Jumps Explain Electromagnetic Spectrum.