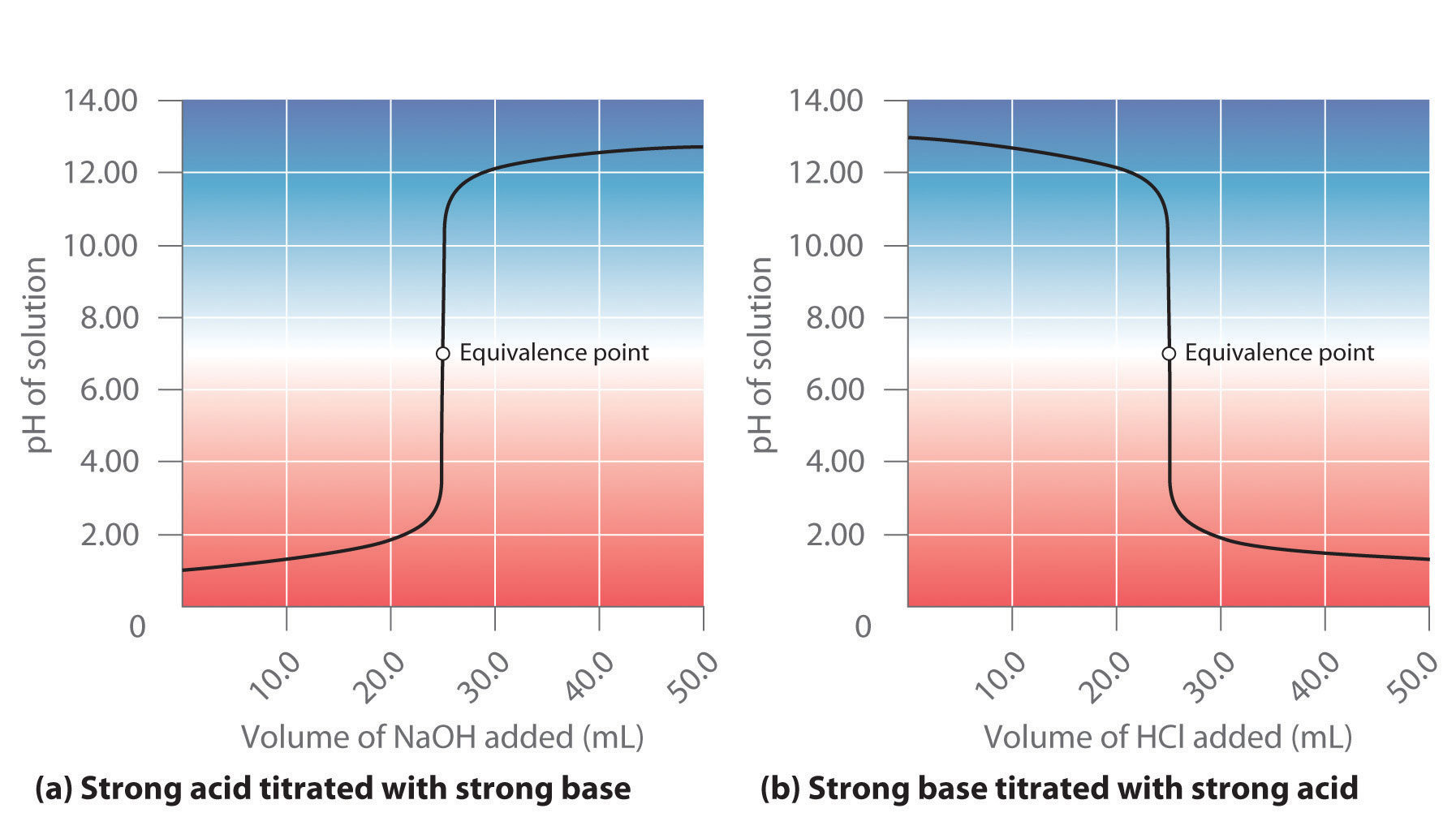
Acid Base Titration End Point . For a ph titration (between an acid and a base), there are two common ways to find the endpoint: In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. Titrations with a ph probe. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. A titration curve is a plot of some solution property versus the amount of added titrant. A titration’s end point was determined using litmus. Using a ph probe and using a colour indicator. This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques.
from saylordotorg.github.io
Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations with a ph probe. This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. A titration curve is a plot of some solution property versus the amount of added titrant. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. A titration’s end point was determined using litmus. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection.
AcidBase Titrations
Acid Base Titration End Point Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration’s end point was determined using litmus. Using a ph probe and using a colour indicator. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Titrations with a ph probe. This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. A titration curve is a plot of some solution property versus the amount of added titrant. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o.
From cameramath.com
[Solved] 4.5 points For the following question, select all that are Acid Base Titration End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations with a ph probe. A titration curve is a plot of some solution property versus the amount of added titrant. Using a ph probe and using a colour indicator. This type of titration serves as a foundational model due to. Acid Base Titration End Point.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Acid Base Titration End Point Titrations with a ph probe. This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. Hna + noh− → na−. Acid Base Titration End Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Acid Base Titration End Point A titration’s end point was determined using litmus. Titrations with a ph probe. A titration curve is a plot of some solution property versus the amount of added titrant. Using a ph probe and using a colour indicator. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the. Acid Base Titration End Point.
From www.chegg.com
Solved 1. Figure 1 shows the titration curves of four Acid Base Titration End Point Titrations with a ph probe. A titration curve is a plot of some solution property versus the amount of added titrant. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. The weak acid titration curve in figure 14.20 shows that only one of the three indicators. Acid Base Titration End Point.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Acid Base Titration End Point The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. A titration’s end point was determined using litmus. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. Titrations with. Acid Base Titration End Point.
From www.slideshare.net
Acid base titration Acid Base Titration End Point In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. A titration’s end point was determined using litmus. A titration curve is a plot of. Acid Base Titration End Point.
From www.chegg.com
Solved The point in the titration when the number of moles Acid Base Titration End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration’s end point was determined using litmus. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. In its simplest form, titration is carried out by measuring the. Acid Base Titration End Point.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Acid Base Titration End Point In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making. Acid Base Titration End Point.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration End Point This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. A titration curve is a plot of some solution property versus the amount of added titrant. A titration’s end point was determined using litmus. Using a ph probe and using a colour. Acid Base Titration End Point.
From mungfali.com
Acid Base Titration Calculation Acid Base Titration End Point A titration’s end point was determined using litmus. Using a ph probe and using a colour indicator. A titration curve is a plot of some solution property versus the amount of added titrant. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: This type of titration serves as a foundational. Acid Base Titration End Point.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Acid Base Titration End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration curve is a plot of some solution property versus the amount of added titrant. Titrations with a ph probe. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to. Acid Base Titration End Point.
From mavink.com
Acid Base Titration Curve Acid Base Titration End Point This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. A titration’s end point was determined using litmus. Hna +. Acid Base Titration End Point.
From morioh.com
Acid Base Titration Curves PH Calculations Acid Base Titration End Point Using a ph probe and using a colour indicator. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. A titration’s end point was determined using litmus. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Hna +. Acid Base Titration End Point.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration End Point A titration’s end point was determined using litmus. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Using a ph probe and. Acid Base Titration End Point.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Acid Base Titration End Point A titration’s end point was determined using litmus. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: In its simplest form, titration is carried out by. Acid Base Titration End Point.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration End Point This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. For a ph titration (between an acid and. Acid Base Titration End Point.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube Acid Base Titration End Point A titration’s end point was determined using litmus. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. Titrations with a ph probe. This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point. Acid Base Titration End Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Acid Base Titration End Point Using a ph probe and using a colour indicator. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. A. Acid Base Titration End Point.
From uwaterloo.ca
Classic acid and base titration in pink Chem 13 News Magazine Acid Base Titration End Point Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations with a ph probe. A titration curve is a plot of some solution property versus the amount of added titrant. In its simplest form, titration is carried out by measuring the. Acid Base Titration End Point.
From www.chegg.com
Solved What point of an acidbase titration has been reached Acid Base Titration End Point This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Using a ph probe and using a colour indicator.. Acid Base Titration End Point.
From mavink.com
Acid Base Titration Curve Acid Base Titration End Point A titration curve is a plot of some solution property versus the amount of added titrant. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. In its simplest form, titration is carried out by measuring the volume of the solution of. Acid Base Titration End Point.
From www.examples.com
Titrations(Notes & Practice Questions) MCAT Examples Acid Base Titration End Point The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. Titrations with a ph probe. This type of titration serves as a foundational model. Acid Base Titration End Point.
From www.sliderbase.com
Titration Acid Base Titration End Point The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. Titrations with a ph probe. A titration’s end point was determined using litmus. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. For a. Acid Base Titration End Point.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Acid Base Titration End Point The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. A titration curve is a plot of some solution property versus the amount of added titrant. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the. Acid Base Titration End Point.
From www.careerstoday.in
Acid Base Titration Careers Today Acid Base Titration End Point Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. For a ph titration (between an acid and a base), there are two common. Acid Base Titration End Point.
From www.slideserve.com
PPT AcidBase Titrations PowerPoint Presentation, free download ID Acid Base Titration End Point For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. A titration curve is a plot of some solution property versus the amount of added titrant. In its simplest form, titration is carried out by measuring the volume of the solution of. Acid Base Titration End Point.
From schoolbag.info
Figure 10.9. Monoprotic Strong Acid and Strong Base Titration Curve A Acid Base Titration End Point In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. A titration curve is a plot of some solution property versus the amount of added titrant. Using a ph probe and using a colour indicator. This type of titration serves as a foundational model due to its. Acid Base Titration End Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Acid Base Titration End Point In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. A titration curve is a plot of some solution property versus the amount of added titrant. A titration’s end point was determined using litmus. This type of titration serves as a foundational model due to its clear. Acid Base Titration End Point.
From saylordotorg.github.io
AcidBase Titrations Acid Base Titration End Point This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. The weak acid titration curve in figure 14.20. Acid Base Titration End Point.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Acid Base Titration End Point Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: A titration curve is a plot of some solution property versus the amount of added titrant. Titrations. Acid Base Titration End Point.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals Acid Base Titration End Point Titrations with a ph probe. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. A titration curve is a plot of some. Acid Base Titration End Point.
From webmis.highland.cc.il.us
AcidBase Titrations Acid Base Titration End Point In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. Titrations with a ph probe. A titration curve is a plot of some. Acid Base Titration End Point.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Acid Base Titration End Point The weak acid titration curve in figure 14.20 shows that only one of the three indicators is suitable for end point detection. In its simplest form, titration is carried out by measuring the volume of the solution of strong base required to complete the reaction. A titration curve is a plot of some solution property versus the amount of added. Acid Base Titration End Point.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Acid Base Titration End Point Hna + noh− → na− + nh2o (13.5.1) (13.5.1) h na + n oh − → n a − + n h 2 o. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The weak acid titration curve in figure 14.20 shows that only one of the three indicators is. Acid Base Titration End Point.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Acid Base Titration End Point A titration’s end point was determined using litmus. Using a ph probe and using a colour indicator. This type of titration serves as a foundational model due to its clear endpoint and predictable outcomes, making it a common starting point for students studying titration techniques. In its simplest form, titration is carried out by measuring the volume of the solution. Acid Base Titration End Point.