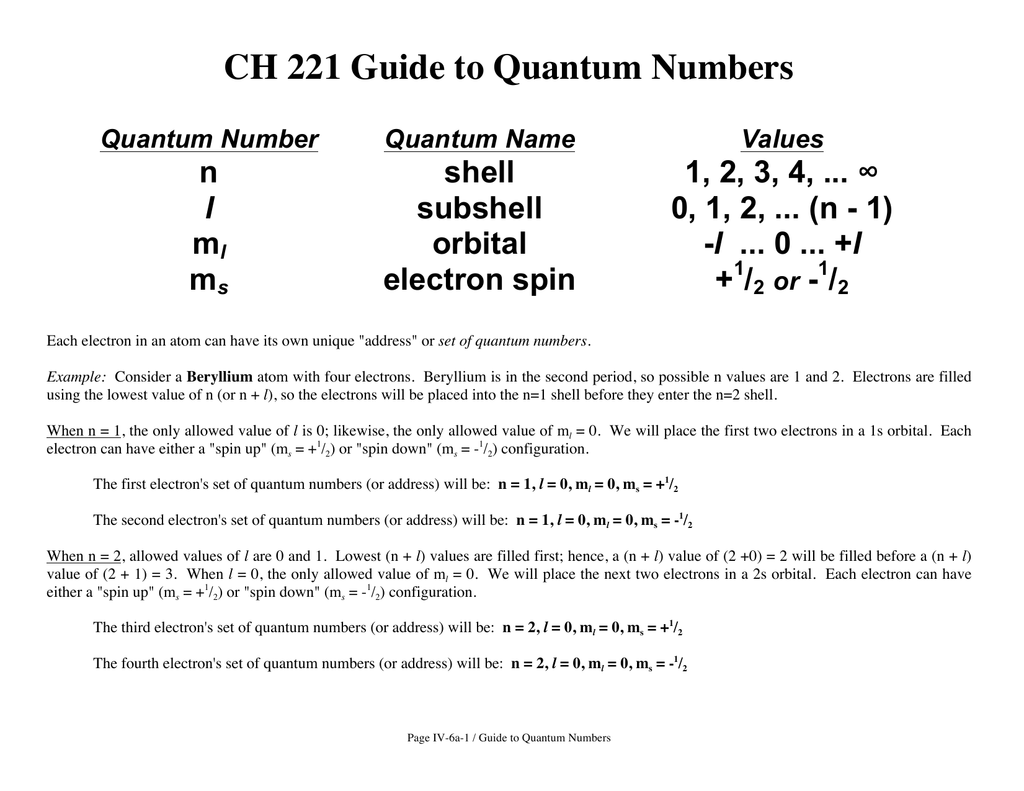
Briefly Explain Quantum Numbers . It is used to project the spin angular momentum along a specific axis. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. An orbital is, so to speak, a house where the electron resides. Because n describes the most probable distance of the. Spin quantum number (m s) the spin quantum number represents the electron’s spin. This is the same as the energy level of the electron. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. The bigger the orbital, the greater the distance of the. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. The allowed values of l z are. In other words, they must. L − 1, l, 30.44.
from studylib.net
An orbital is, so to speak, a house where the electron resides. In other words, they must. L − 1, l, 30.44. Because n describes the most probable distance of the. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. The bigger the orbital, the greater the distance of the. It is used to project the spin angular momentum along a specific axis. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell.
Quantum Numbers
Briefly Explain Quantum Numbers It is used to project the spin angular momentum along a specific axis. Spin quantum number (m s) the spin quantum number represents the electron’s spin. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. This is the same as the energy level of the electron. The bigger the orbital, the greater the distance of the. It is used to project the spin angular momentum along a specific axis. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. The allowed values of l z are. An orbital is, so to speak, a house where the electron resides. L − 1, l, 30.44. In other words, they must. Because n describes the most probable distance of the. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does.
From brainly.in
what are the 4 quantum numbers....explain briefly? Brainly.in Briefly Explain Quantum Numbers L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. L − 1, l, 30.44. This is the same as the energy level of the electron. The bigger the orbital, the greater the distance of the. Because n describes the most probable distance of the. The principal quantum number (\(n\)). Briefly Explain Quantum Numbers.
From deepstash.com
Spin Quantum Numbers (ms) Deepstash Briefly Explain Quantum Numbers The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. Because n describes the most probable distance of the. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. This is the same as the energy level of the electron. L z = m l h. Briefly Explain Quantum Numbers.
From stanley-owncreator.blogspot.com
Explain Different Types of Quantum Numbers Briefly Explain Quantum Numbers The allowed values of l z are. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. This is the same as the energy level of the electron. The bigger the orbital, the greater the distance of the. Spin quantum number (m s) the spin quantum number represents the electron’s. Briefly Explain Quantum Numbers.
From slideplayer.com
Chapter 13 Notes Electrons in an Atom. ppt download Briefly Explain Quantum Numbers Because n describes the most probable distance of the. Spin quantum number (m s) the spin quantum number represents the electron’s spin. L − 1, l, 30.44. The allowed values of l z are. This is the same as the energy level of the electron. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell.. Briefly Explain Quantum Numbers.
From byjus.com
explain principal azimuthal and spin quantum numbers Briefly Explain Quantum Numbers L − 1, l, 30.44. In other words, they must. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. The allowed values of l z are. The principal quantum number. Briefly Explain Quantum Numbers.
From gamesmartz.com
Quantum Number Definition & Image GameSmartz Briefly Explain Quantum Numbers L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. Spin quantum number (m s) the spin quantum number represents the electron’s spin. It is used to project the spin angular momentum along a specific axis. In other words, they must. L − 1, l, 30.44. The values of quantized. Briefly Explain Quantum Numbers.
From mavink.com
Quantum Numbers Symbols Briefly Explain Quantum Numbers The allowed values of l z are. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. An orbital is, so to speak, a house where the electron resides. The bigger the orbital, the greater the distance of the. This is. Briefly Explain Quantum Numbers.
From slideplayer.com
Quantum Numbers specify the properties of atomic orbitals and their Briefly Explain Quantum Numbers An orbital is, so to speak, a house where the electron resides. Because n describes the most probable distance of the. It is used to project the spin angular momentum along a specific axis. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature. Briefly Explain Quantum Numbers.
From stock.adobe.com
Quantum numbers physics vector illustration infographic Stock Vector Briefly Explain Quantum Numbers The allowed values of l z are. It is used to project the spin angular momentum along a specific axis. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. L − 1, l, 30.44. In. Briefly Explain Quantum Numbers.
From www.numerade.com
Give the allowable combinations of quantum numbers for each of the Briefly Explain Quantum Numbers It is used to project the spin angular momentum along a specific axis. L − 1, l, 30.44. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. The bigger the orbital, the greater the distance of the. Spin quantum number (m s) the spin quantum number represents the electron’s spin.. Briefly Explain Quantum Numbers.
From ar.inspiredpencil.com
Quantum Numbers Diagram Briefly Explain Quantum Numbers L − 1, l, 30.44. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. Because n describes the most probable distance. Briefly Explain Quantum Numbers.
From freeonlinehelpns.blogspot.com
Free Online Help explain type of quantum number , principle quantum Briefly Explain Quantum Numbers An orbital is, so to speak, a house where the electron resides. The bigger the orbital, the greater the distance of the. The allowed values of l z are. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. It is used to project the spin angular momentum along a. Briefly Explain Quantum Numbers.
From www.youtube.com
Quantum numbers Four Types of Quantum Numbers Basics ViVid Chemie YouTube Briefly Explain Quantum Numbers The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. It is used to project the spin angular momentum along a specific axis. In other words, they must. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. The bigger the orbital, the greater the distance. Briefly Explain Quantum Numbers.
From askfilo.com
What are quantum numbers. Explain the significance of these quantum numbe.. Briefly Explain Quantum Numbers Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. An orbital is, so to speak, a house where the electron resides. Spin quantum number (m s) the spin quantum number represents the electron’s spin. Because n describes the most probable distance of the. L z = m l h 2π. Briefly Explain Quantum Numbers.
From studylib.net
Lecture 4 Quantum Numbers Briefly Explain Quantum Numbers Spin quantum number (m s) the spin quantum number represents the electron’s spin. In other words, they must. This is the same as the energy level of the electron. An orbital is, so to speak, a house where the electron resides. The bigger the orbital, the greater the distance of the. The values of quantized entities are expressed in terms. Briefly Explain Quantum Numbers.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs Briefly Explain Quantum Numbers It is used to project the spin angular momentum along a specific axis. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. The bigger the orbital, the greater the distance of the. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. L − 1,. Briefly Explain Quantum Numbers.
From www.youtube.com
Quantum Numbers Azimuthal Quantum Number Quantum Spin Briefly Explain Quantum Numbers An orbital is, so to speak, a house where the electron resides. L − 1, l, 30.44. In other words, they must. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. Spin quantum number (m s) the spin quantum number represents the electron’s spin. This is the same as the. Briefly Explain Quantum Numbers.
From chemistrypubs.com
Quantum Numbers Principle, Examples, Significance Chemistrupubs Briefly Explain Quantum Numbers In other words, they must. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. It is used to project the spin angular momentum along a specific axis. L z = m l h 2π m l = − l, −. Briefly Explain Quantum Numbers.
From www.biologystudypoint.com
Quantum Number Types, Definition, Example 2024 Briefly Explain Quantum Numbers It is used to project the spin angular momentum along a specific axis. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. This is the same as the energy level of the electron. L − 1, l, 30.44. In other words, they must. The values of quantized entities are expressed in terms of quantum. Briefly Explain Quantum Numbers.
From brainly.in
Find the definition Of a quantum number?How many types of quantum Briefly Explain Quantum Numbers The bigger the orbital, the greater the distance of the. In other words, they must. Spin quantum number (m s) the spin quantum number represents the electron’s spin. This is the same as the energy level of the electron. The allowed values of l z are. L − 1, l, 30.44. It is used to project the spin angular momentum. Briefly Explain Quantum Numbers.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition Briefly Explain Quantum Numbers Spin quantum number (m s) the spin quantum number represents the electron’s spin. Because n describes the most probable distance of the. An orbital is, so to speak, a house where the electron resides. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. L − 1, l, 30.44. Only. Briefly Explain Quantum Numbers.
From www.freepik.com
Premium Vector Principle quantum number vector illustration graphic Briefly Explain Quantum Numbers The bigger the orbital, the greater the distance of the. Spin quantum number (m s) the spin quantum number represents the electron’s spin. L − 1, l, 30.44. In other words, they must. An orbital is, so to speak, a house where the electron resides. The values of quantized entities are expressed in terms of quantum numbers, and the rules. Briefly Explain Quantum Numbers.
From hubpages.com
Atoms and Atomic Structure HubPages Briefly Explain Quantum Numbers It is used to project the spin angular momentum along a specific axis. The bigger the orbital, the greater the distance of the. An orbital is, so to speak, a house where the electron resides. The allowed values of l z are. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are. Briefly Explain Quantum Numbers.
From byjus.com
explain principal azimuthal and spin quantum numbers Briefly Explain Quantum Numbers An orbital is, so to speak, a house where the electron resides. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. It is used to project the spin angular momentum along a specific axis. The bigger the orbital, the greater the distance of the. This is the same as the energy level of the. Briefly Explain Quantum Numbers.
From askfilo.com
What are quantum Numbers? Briefly describe all the four quantum nos (2) Briefly Explain Quantum Numbers It is used to project the spin angular momentum along a specific axis. The bigger the orbital, the greater the distance of the. In other words, they must. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. L − 1,. Briefly Explain Quantum Numbers.
From socratic.org
What do the four quantum numbers describe about an electron? Socratic Briefly Explain Quantum Numbers Because n describes the most probable distance of the. It is used to project the spin angular momentum along a specific axis. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. The allowed values of l z are. In other words, they must. This is the same as the. Briefly Explain Quantum Numbers.
From studylib.net
Quantum Numbers Briefly Explain Quantum Numbers The bigger the orbital, the greater the distance of the. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. The allowed values of l z are. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. The values of quantized entities are expressed in. Briefly Explain Quantum Numbers.
From www.freeastroscience.com
What are quantum numbers? Briefly Explain Quantum Numbers The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. The bigger the orbital, the greater the distance of the. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers m s. This is. Briefly Explain Quantum Numbers.
From www.youtube.com
Quantum numbers Electronic structure of atoms Chemistry Khan Briefly Explain Quantum Numbers The bigger the orbital, the greater the distance of the. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. Because n describes the most probable distance of the. The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. Only two electrons can occupy an. Briefly Explain Quantum Numbers.
From pnabeat.weebly.com
How do quantum numbers help chemists pnabeat Briefly Explain Quantum Numbers The bigger the orbital, the greater the distance of the. Spin quantum number (m s) the spin quantum number represents the electron’s spin. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. Only two electrons can occupy an orbital, and they must do so with opposite spin quantum numbers. Briefly Explain Quantum Numbers.
From www.youtube.com
Quantum Numbers Principal Quantum Number Chapter 2 Class 11th Briefly Explain Quantum Numbers This is the same as the energy level of the electron. The bigger the orbital, the greater the distance of the. The allowed values of l z are. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. It is used to project the spin angular momentum along a specific. Briefly Explain Quantum Numbers.
From www.doubtnut.com
[Odia] What are quantum numbers ? Describe briefly four quantum number Briefly Explain Quantum Numbers Because n describes the most probable distance of the. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. The bigger the orbital, the greater the distance of the. An orbital is, so to speak, a house where the electron resides.. Briefly Explain Quantum Numbers.
From www.slideserve.com
PPT What are the Types of Quantum Numbers? PowerPoint Presentation Briefly Explain Quantum Numbers It is used to project the spin angular momentum along a specific axis. L − 1, l, 30.44. L z = m l h 2π m l = − l, − l + 1,., − 1, 0, 1,. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance. Briefly Explain Quantum Numbers.
From www.youtube.com
Quantum Numbers, Principal quantum numbers, Azimuthal and spin quantum Briefly Explain Quantum Numbers L − 1, l, 30.44. Because n describes the most probable distance of the. The values of quantized entities are expressed in terms of quantum numbers, and the rules governing them are of the utmost importance in determining what nature is and does. L z = m l h 2π m l = − l, − l + 1,., −. Briefly Explain Quantum Numbers.
From www.studypool.com
SOLUTION Principle quantum numbers and azimuthal quantum number Briefly Explain Quantum Numbers The principal quantum number (\(n\)) the principal quantum number, \(n\), designates the principal electron shell. The allowed values of l z are. It is used to project the spin angular momentum along a specific axis. The bigger the orbital, the greater the distance of the. L z = m l h 2π m l = − l, − l +. Briefly Explain Quantum Numbers.