How To Make Dilution Of Solution . Ml of a 2.0 m solution of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to. Preparing dilutions is a common activity in the chemistry lab and elsewhere. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. Once you understand this relationship, the calculations are simple. To dilute a solution means to add more solvent without the addition of more solute.
from www.youtube.com
Once you understand this relationship, the calculations are simple. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Ml of a 2.0 m solution of. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. Preparing dilutions is a common activity in the chemistry lab and elsewhere. To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume.
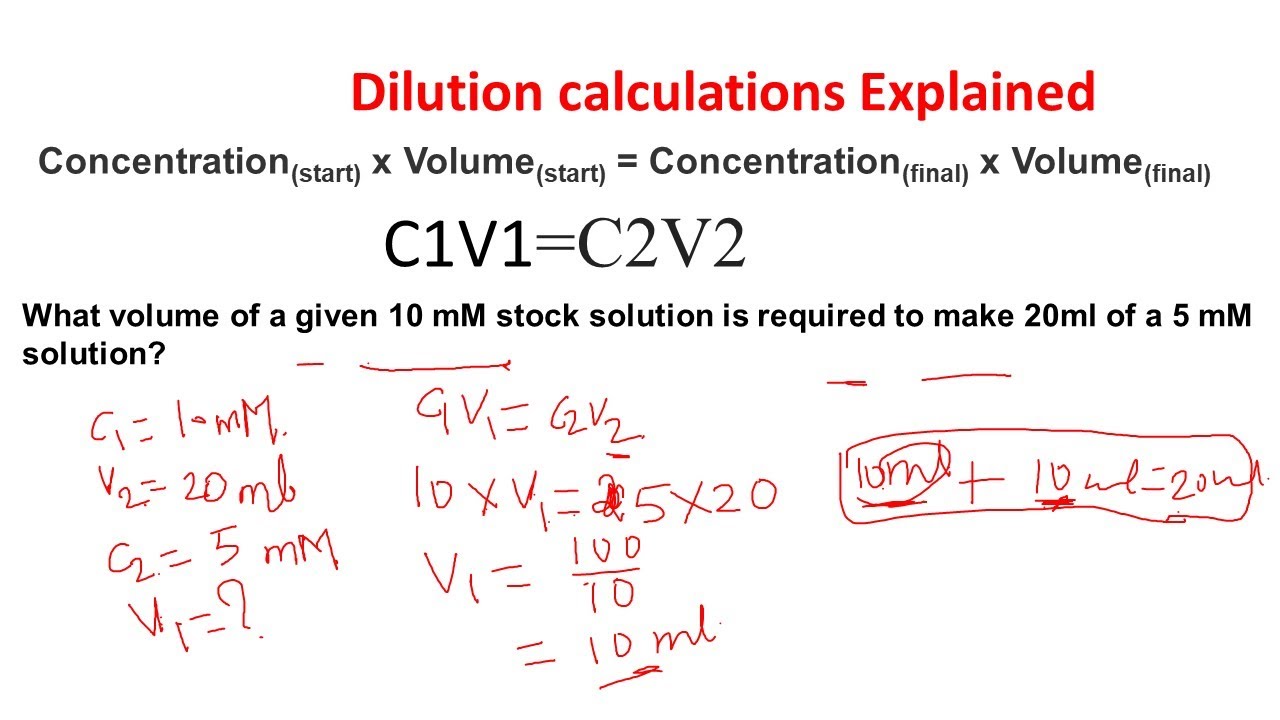
Dilution calculations Dilution problems Stock dilutions Biology and
How To Make Dilution Of Solution Preparing dilutions is a common activity in the chemistry lab and elsewhere. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Ml of a 2.0 m solution of. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. Once you understand this relationship, the calculations are simple. Preparing dilutions is a common activity in the chemistry lab and elsewhere. State whether the concentration of a solution is directly or indirectly proportional to its volume. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water.
From recipestime.com
How to Make Your Own Disinfecting Wipes Recipes Time How To Make Dilution Of Solution State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to. Once you understand this relationship, the calculations are simple. To dilute a solution means to add more solvent without the addition of more solute. When you know all four values in the equation c. How To Make Dilution Of Solution.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts How To Make Dilution Of Solution Preparing dilutions is a common activity in the chemistry lab and elsewhere. Once you understand this relationship, the calculations are simple. Of course, the resulting solution is thoroughly mixed so as to. Ml of a 2.0 m solution of. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the.. How To Make Dilution Of Solution.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina How To Make Dilution Of Solution Ml of a 2.0 m solution of. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. Preparing dilutions is a common activity in the chemistry lab and. How To Make Dilution Of Solution.
From spmchemistry.blog.onlinetuition.com.my
Dilution SPM Chemistry How To Make Dilution Of Solution Once you understand this relationship, the calculations are simple. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. Preparing dilutions is a common activity in the chemistry. How To Make Dilution Of Solution.
From www.facebook.com
🌟 Unlock the Power of Knowledge with Afrivet Academy! 🌾 🐄🌿 **Knowing How To Make Dilution Of Solution To dilute a solution means to add more solvent without the addition of more solute. Of course, the resulting solution is thoroughly mixed so as to. Preparing dilutions is a common activity in the chemistry lab and elsewhere. Ml of a 2.0 m solution of. Pour the stock solution into a graduated cylinder or other measuring container large enough to. How To Make Dilution Of Solution.
From joivmtjnj.blob.core.windows.net
How To Make Dilution From Stock Solution at Dorothy Grenier blog How To Make Dilution Of Solution When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. Ml of a 2.0 m solution of. Pour the. How To Make Dilution Of Solution.
From groundhogapps.com
Unlocking Efficiency Ore Dilution and Quality Control in OpenPit How To Make Dilution Of Solution To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. Once you understand this relationship, the calculations are simple. Ml of a 2.0 m solution of. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final. How To Make Dilution Of Solution.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in How To Make Dilution Of Solution Ml of a 2.0 m solution of. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. Once you understand this relationship, the. How To Make Dilution Of Solution.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality How To Make Dilution Of Solution To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. Ml of a 2.0 m solution of. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. State whether the concentration of a. How To Make Dilution Of Solution.
From www.facebook.com
🌟 Unlock the Power of Knowledge with Afrivet Academy! 🌾 🐄🌿 **Knowing How To Make Dilution Of Solution To dilute a solution means to add more solvent without the addition of more solute. Once you understand this relationship, the calculations are simple. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. Pour the stock solution into a graduated cylinder or other. How To Make Dilution Of Solution.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube How To Make Dilution Of Solution State whether the concentration of a solution is directly or indirectly proportional to its volume. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml. How To Make Dilution Of Solution.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube How To Make Dilution Of Solution When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. To dilute a solution means to add more solvent without the addition of more solute. Ml of a 2.0 m solution of. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167. How To Make Dilution Of Solution.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and How To Make Dilution Of Solution Once you understand this relationship, the calculations are simple. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Of course, the resulting solution is thoroughly mixed so as to. Ml of a 2.0 m solution of. State whether the concentration of a solution is directly or indirectly proportional. How To Make Dilution Of Solution.
From www.youtube.com
How to Make a Dilution of a Stock Solution YouTube How To Make Dilution Of Solution When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Of course, the resulting solution is thoroughly mixed so as to. Once you understand this relationship, the calculations are simple. Ml of a 2.0 m solution of. Preparing dilutions is a common activity in the chemistry lab and elsewhere.. How To Make Dilution Of Solution.
From www.integra-biosciences.com
How to do serial dilutions (including calculations) INTEGRA How To Make Dilution Of Solution To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. State whether the concentration of a solution is directly or indirectly proportional to. How To Make Dilution Of Solution.
From www.youtube.com
Stock Solution Dilutions Dilution Calculation [Learn how to make any How To Make Dilution Of Solution State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. Preparing dilutions is a. How To Make Dilution Of Solution.
From www.gauthmath.com
Solved 15 To make 100mL of 5mg/mL using a stock solution of 50mg/mL How To Make Dilution Of Solution State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to. Ml of a 2.0 m solution of. Preparing dilutions is a common activity in the chemistry lab and elsewhere. When you know all four values in the equation c 1 v 1 = c. How To Make Dilution Of Solution.
From carlosgokeowen.blogspot.com
What is Dilution How To Make Dilution Of Solution State whether the concentration of a solution is directly or indirectly proportional to its volume. Ml of a 2.0 m solution of. To dilute a solution means to add more solvent without the addition of more solute. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833. How To Make Dilution Of Solution.
From mrkhemistry.com
Buffer solutions dilution what effects will it have on pH? How To Make Dilution Of Solution Ml of a 2.0 m solution of. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. To dilute a solution means to add more solvent without the. How To Make Dilution Of Solution.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision How To Make Dilution Of Solution Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. Once you understand this relationship, the calculations are simple. Ml of a 2.0 m solution of. Preparing dilutions is a common activity in the chemistry lab and elsewhere. Of course, the resulting solution is thoroughly mixed so as to.. How To Make Dilution Of Solution.
From www.chegg.com
Solved Serial dilution is a common technique used in How To Make Dilution Of Solution Preparing dilutions is a common activity in the chemistry lab and elsewhere. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition of more solute. Once you understand this relationship, the calculations are simple. To make a liter of dilution with a solution. How To Make Dilution Of Solution.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts How To Make Dilution Of Solution When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Of course, the resulting solution is thoroughly mixed so as to. Ml of a 2.0 m solution of. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the.. How To Make Dilution Of Solution.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation How To Make Dilution Of Solution Ml of a 2.0 m solution of. To dilute a solution means to add more solvent without the addition of more solute. Preparing dilutions is a common activity in the chemistry lab and elsewhere. Once you understand this relationship, the calculations are simple. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167. How To Make Dilution Of Solution.
From www.facebook.com
🌟 Unlock the Power of Knowledge with Afrivet Academy! 🌾 🐄🌿 **Knowing How To Make Dilution Of Solution When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. Preparing dilutions is a common activity in the chemistry lab and elsewhere. Once you understand this relationship, the. How To Make Dilution Of Solution.
From www.pinterest.com.mx
Dilution when solvent is added to dilute a solution, the number of How To Make Dilution Of Solution To dilute a solution means to add more solvent without the addition of more solute. Once you understand this relationship, the calculations are simple. Ml of a 2.0 m solution of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Of course, the resulting solution is thoroughly mixed so as to. When you know. How To Make Dilution Of Solution.
From exoyaaeul.blob.core.windows.net
Dilution Definition In A Sentence at Tomas Branson blog How To Make Dilution Of Solution To dilute a solution means to add more solvent without the addition of more solute. Ml of a 2.0 m solution of. Preparing dilutions is a common activity in the chemistry lab and elsewhere. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. To make a liter of. How To Make Dilution Of Solution.
From davinawellness.com
Dilution Davina Wellness How To Make Dilution Of Solution Preparing dilutions is a common activity in the chemistry lab and elsewhere. Of course, the resulting solution is thoroughly mixed so as to. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. State whether the concentration of a solution is directly or indirectly proportional to its volume. When. How To Make Dilution Of Solution.
From sciencequery.com
What is serial dilution method? And how to calculate? Science Query How To Make Dilution Of Solution To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. Once you understand this relationship, the calculations are simple. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. Of course, the resulting. How To Make Dilution Of Solution.
From www.myxxgirl.com
Serial Dilution Of An Initial Sample Or Culture To Obtain Solutions How To Make Dilution Of Solution When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Ml of a 2.0 m solution of. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume of the. State whether the concentration of a solution is directly or indirectly. How To Make Dilution Of Solution.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY How To Make Dilution Of Solution Of course, the resulting solution is thoroughly mixed so as to. Once you understand this relationship, the calculations are simple. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Pour the stock solution into a graduated cylinder or other measuring container large enough to hold the final volume. How To Make Dilution Of Solution.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory How To Make Dilution Of Solution When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Ml of a 2.0 m solution of. To dilute a solution means to add more solvent without the addition of more solute. State whether the concentration of a solution is directly or indirectly proportional to its volume. Preparing dilutions. How To Make Dilution Of Solution.
From www.youtube.com
How to make dilutions in lab (110,120,150,1100,1200),easy way to How To Make Dilution Of Solution Of course, the resulting solution is thoroughly mixed so as to. State whether the concentration of a solution is directly or indirectly proportional to its volume. Once you understand this relationship, the calculations are simple. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Pour the stock solution. How To Make Dilution Of Solution.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii How To Make Dilution Of Solution Ml of a 2.0 m solution of. To dilute a solution means to add more solvent without the addition of more solute. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml of water. Of course, the resulting solution is thoroughly mixed so as to. Once. How To Make Dilution Of Solution.
From loelxqvfe.blob.core.windows.net
How To Calculate The Total Dilution Factor at Jason Briggs blog How To Make Dilution Of Solution To dilute a solution means to add more solvent without the addition of more solute. When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. To make a liter of dilution with a solution ratio of 1:5, you'll need to mix 167 ml of stock solution in 833 ml. How To Make Dilution Of Solution.
From www.facebook.com
🌟 Unlock the Power of Knowledge with Afrivet Academy! 🌾 🐄🌿 **Knowing How To Make Dilution Of Solution When you know all four values in the equation c 1 v 1 = c 2 v 2, perform your dilution. Preparing dilutions is a common activity in the chemistry lab and elsewhere. State whether the concentration of a solution is directly or indirectly proportional to its volume. To dilute a solution means to add more solvent without the addition. How To Make Dilution Of Solution.