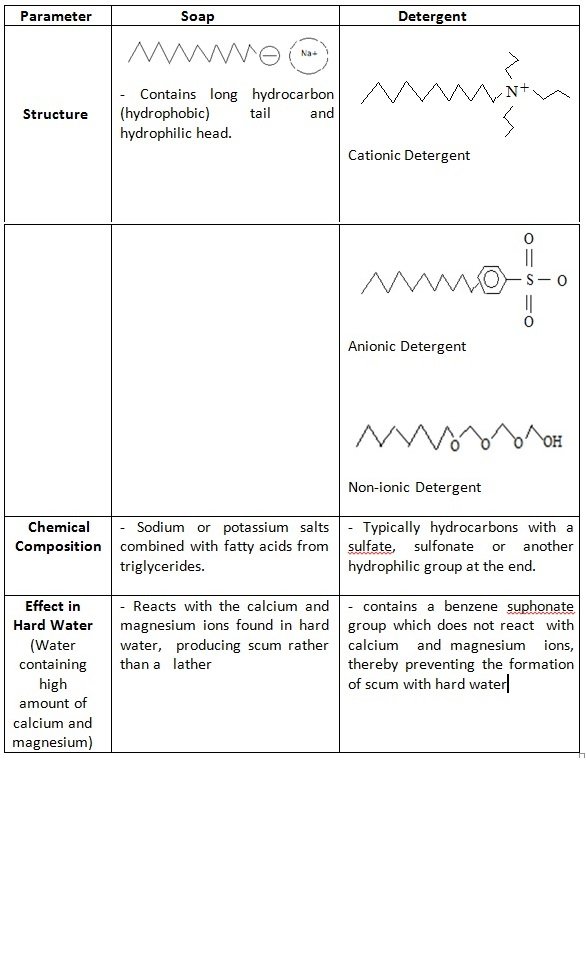
Soap Example Chemistry . examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). The use of such compounds as cleaning agents is. Soap is a classic cleaning agent that has been used for centuries. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also.
from easychem.com.au
alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. The use of such compounds as cleaning agents is. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap is a classic cleaning agent that has been used for centuries.
The Difference Between Soaps And Synthetic Detergents EasyChem Australia
Soap Example Chemistry today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. Soap is a classic cleaning agent that has been used for centuries. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The use of such compounds as cleaning agents is. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also.
From study.com
Soap & Detergent Chemistry, Types & Differences Lesson Soap Example Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character. Soap Example Chemistry.
From www.slideshare.net
Chemistry of soaps Soap Example Chemistry soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. alkali metal salts of fatty acids are more soluble in water than the acids. Soap Example Chemistry.
From brainly.in
what is the difference between the molecules of soap and detergents Soap Example Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge. Soap Example Chemistry.
From www.shutterstock.com
Soap Chemistry Soap Chemistry Formula Structure Stock Vector (Royalty Soap Example Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). Soap is a classic cleaning agent that has been used for centuries. today, detergents are more likely to be a mixture of synthetic. Soap Example Chemistry.
From thesoapmoleculeco.com
The Soap Molecule Co. Soap Example Chemistry alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in. Soap Example Chemistry.
From www.sharpestarena.com
Production of Soap Complete Project on Soap Making Soap Example Chemistry soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is a classic cleaning agent that has been used for centuries. The use of such compounds as cleaning agents is. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. examples. Soap Example Chemistry.
From bossanovasoap.com
CHEMISTRY Soap Example Chemistry Soap is a classic cleaning agent that has been used for centuries. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). Its fundamental chemistry involves the combination of fats or oils with an. Soap Example Chemistry.
From www.pinterest.com
Saponification Soap making, Chemistry, Soap Soap Example Chemistry today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. Soap is a classic cleaning agent that has been used for centuries. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Its fundamental chemistry. Soap Example Chemistry.
From studylib.net
Chemical Reactions Soap Making Soap Example Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The use of such compounds as cleaning agents is. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. alkali metal. Soap Example Chemistry.
From www.japudo.com.br
Chemistry Roberto Akira Soap Example Chemistry alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. revise the action of soaps and detergents for higher chemistry, and. Soap Example Chemistry.
From lilis-chemistryblog.weebly.com
Soap Making Chemistry Blog Soap Example Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. The use of such compounds as cleaning agents is. soaps are. Soap Example Chemistry.
From www.zensoaps.com
Chemical Process of Soap Soap Example Chemistry soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is a classic cleaning agent that has been used for centuries. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. today, detergents are more. Soap Example Chemistry.
From www.flinnsci.ca
Making Soap The Oldest Organic Reaction—ChemTopic™ Lab Activity Soap Example Chemistry soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Soap is a classic cleaning agent that has been used for centuries. examples of soap and. Soap Example Chemistry.
From www.teachoo.com
[Class 10] Soaps and Detergents Structure, Cleansing Action and more Soap Example Chemistry revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. The use of such compounds as cleaning agents is. examples of soap and. Soap Example Chemistry.
From www.slideshare.net
Chemistry of soaps Soap Example Chemistry Soap is a classic cleaning agent that has been used for centuries. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also.. Soap Example Chemistry.
From www.slideshare.net
Soap and detergent ( chemistry folio form 5 ) Soap Example Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. alkali metal salts of fatty acids are more soluble in water than the acids. Soap Example Chemistry.
From easychem.com.au
The Difference Between Soaps And Synthetic Detergents EasyChem Australia Soap Example Chemistry examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. alkali metal salts of fatty acids are more soluble. Soap Example Chemistry.
From www.pinterest.com
Saponification explanation. Soap making, Chemistry, Soap Soap Example Chemistry examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. The use of. Soap Example Chemistry.
From www.scribd.com
Soap Soap Chemical Substances Soap Example Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is a classic cleaning agent that has been used for centuries. revise the action of soaps and detergents for higher chemistry,. Soap Example Chemistry.
From www.dreamstime.com
General Formula of Solid and Liquid Soap Molecule. RCOONa, RCOOK Stock Soap Example Chemistry soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. revise the action of soaps and detergents for higher chemistry, and learn about the. Soap Example Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Soap Soap Example Chemistry Soap is a classic cleaning agent that has been used for centuries. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. examples of soap and detergent molecules, are. Soap Example Chemistry.
From www.goodreads.com
Soap Chemistry Discover The Basics Of Soap Chemistry by Wan Yamane Soap Example Chemistry soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The use of such compounds as cleaning agents is. Soap is a classic cleaning agent that has been used for centuries. Its fundamental. Soap Example Chemistry.
From www.slideshare.net
Chemistry of soaps Soap Example Chemistry revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a. Soap Example Chemistry.
From www.slideserve.com
PPT FUNDAMENTALS OF CHEMISTRY PowerPoint Presentation, free download Soap Example Chemistry Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. The use of such compounds as cleaning agents is. revise the. Soap Example Chemistry.
From ar.inspiredpencil.com
Preparation Of Soap Chemistry Soap Example Chemistry revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). Its fundamental. Soap Example Chemistry.
From ar.inspiredpencil.com
Preparation Of Soap Chemistry Soap Example Chemistry today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. Soap is a classic cleaning agent that has been used for centuries. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. examples. Soap Example Chemistry.
From www.thoughtco.com
How Saponification Makes Soap Soap Example Chemistry revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The use of such compounds as cleaning agents is. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Soap is a classic cleaning. Soap Example Chemistry.
From www.pinterest.com
An Introduction to Chemistry Soap, Soap making, Chemical reactions Soap Example Chemistry examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. The use of such compounds as cleaning agents. Soap Example Chemistry.
From brainly.in
What is chemical formula of soap? Brainly.in Soap Example Chemistry soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically. Soap Example Chemistry.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 Soap Example Chemistry Soap is a classic cleaning agent that has been used for centuries. alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). The use of such compounds as cleaning agents is. soaps are. Soap Example Chemistry.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences Soap Example Chemistry Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate 'head'. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. Soap is a classic cleaning agent that has been used for centuries. alkali metal salts of fatty acids are more soluble in. Soap Example Chemistry.
From www.slideshare.net
Soap and detergent ( chemistry folio form 5 ) Soap Example Chemistry alkali metal salts of fatty acids are more soluble in water than the acids themselves, and the amphiphilic character of these substances also. today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike. soaps are sodium or potassium fatty acids salts, produced from the. Soap Example Chemistry.
From www.youtube.com
Chemistry 101 How does soap work? YouTube Soap Example Chemistry examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a chemical reaction called saponification. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. today, detergents are more. Soap Example Chemistry.
From www.slideshare.net
Chemistry of soaps Soap Example Chemistry examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). The use of such compounds as cleaning agents is. revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. soaps are sodium or potassium fatty acids salts, produced from the hydrolysis of fats in a. Soap Example Chemistry.
From www.slideshare.net
How to make soap Experiment for Chemistry lessons Soap Example Chemistry The use of such compounds as cleaning agents is. Its fundamental chemistry involves the combination of fats or oils with an alkaline substance, typically sodium hydroxide (lye) in a process known as saponification. examples of soap and detergent molecules, are shown in figure \(\pageindex{4}\). Each soap molecule has a long hydrocarbon chain, sometimes called its 'tail', with a carboxylate. Soap Example Chemistry.