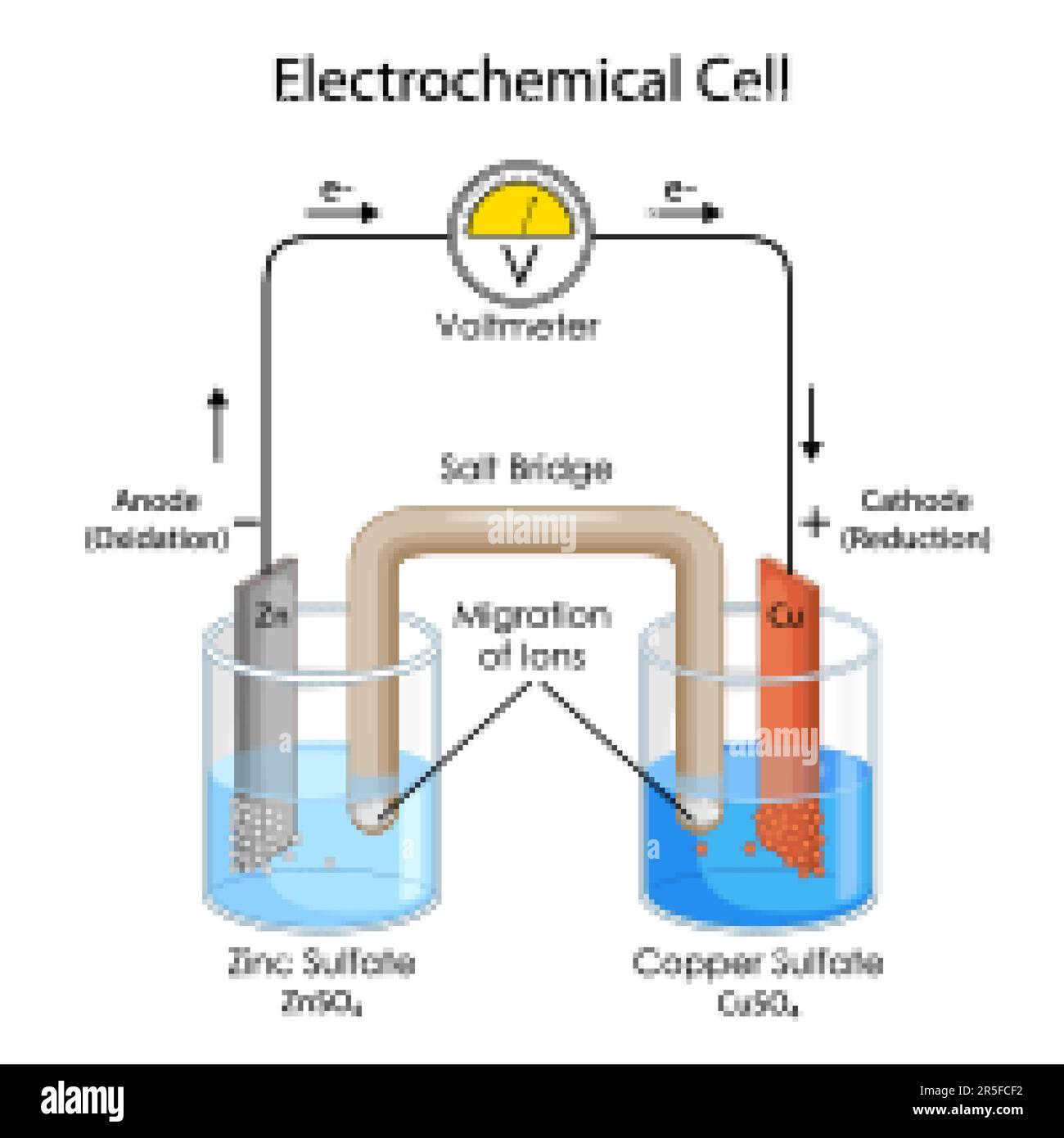
Give Two Examples Of Electrochemical Cell . galvanic cells and electrolytic cells are examples of electrochemical cells. there are two main types of electrochemical cell: Describe two types of situations that would there are two types of electrochemical cells: Galvanic cells and electrolytic cells. What would you put inside a salt bridge? What processes are responsible for conduction of electricity in an electrochemical cell? describe what you know about an electrochemical cell. Galvanic cells are named for the. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Galvanic cells, which are also known as voltaic.
from www.alamy.com
Galvanic cells and electrolytic cells. there are two types of electrochemical cells: there are two main types of electrochemical cell: galvanic cells and electrolytic cells are examples of electrochemical cells. describe what you know about an electrochemical cell. What would you put inside a salt bridge? electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. Galvanic cells are named for the. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Describe two types of situations that would
Educational Diagram of Chart showing Physics concept of Electrochemical
Give Two Examples Of Electrochemical Cell Galvanic cells are named for the. describe what you know about an electrochemical cell. Galvanic cells are named for the. What processes are responsible for conduction of electricity in an electrochemical cell? there are two types of electrochemical cells: in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Galvanic cells and electrolytic cells. there are two main types of electrochemical cell: Describe two types of situations that would galvanic cells and electrolytic cells are examples of electrochemical cells. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. Galvanic cells, which are also known as voltaic. What would you put inside a salt bridge?
From hxeljcwuc.blob.core.windows.net
Electrochemical Cell Real Life Examples at Steven Smith blog Give Two Examples Of Electrochemical Cell galvanic cells and electrolytic cells are examples of electrochemical cells. Galvanic cells and electrolytic cells. What would you put inside a salt bridge? there are two types of electrochemical cells: Describe two types of situations that would What processes are responsible for conduction of electricity in an electrochemical cell? there are two main types of electrochemical cell:. Give Two Examples Of Electrochemical Cell.
From www.studocu.com
Electrochemical CELL Engineering chemistry ELECTROCHEMICAL CELL Give Two Examples Of Electrochemical Cell Describe two types of situations that would galvanic cells and electrolytic cells are examples of electrochemical cells. there are two main types of electrochemical cell: What processes are responsible for conduction of electricity in an electrochemical cell? What would you put inside a salt bridge? Galvanic cells and electrolytic cells. Galvanic cells are named for the. electrochemical. Give Two Examples Of Electrochemical Cell.
From www.collegesearch.in
Electrochemical Cell Definitions, Examples, Electrochemistry Give Two Examples Of Electrochemical Cell What processes are responsible for conduction of electricity in an electrochemical cell? there are two types of electrochemical cells: there are two main types of electrochemical cell: Galvanic cells and electrolytic cells. galvanic cells and electrolytic cells are examples of electrochemical cells. Describe two types of situations that would in an electrochemical cell, we allow electrons. Give Two Examples Of Electrochemical Cell.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Give Two Examples Of Electrochemical Cell there are two main types of electrochemical cell: What would you put inside a salt bridge? Galvanic cells are named for the. there are two types of electrochemical cells: Describe two types of situations that would galvanic cells and electrolytic cells are examples of electrochemical cells. in an electrochemical cell, we allow electrons to be transferred,. Give Two Examples Of Electrochemical Cell.
From www.youtube.com
Electrochemistry 04 Writing Electrochemical Cell Notation YouTube Give Two Examples Of Electrochemical Cell there are two main types of electrochemical cell: galvanic cells and electrolytic cells are examples of electrochemical cells. Galvanic cells and electrolytic cells. Describe two types of situations that would Galvanic cells, which are also known as voltaic. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. What. Give Two Examples Of Electrochemical Cell.
From www.expii.com
Electrochemical Cell — Definition & Overview Expii Give Two Examples Of Electrochemical Cell there are two main types of electrochemical cell: there are two types of electrochemical cells: in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Galvanic cells, which are also known as voltaic. Galvanic cells are named for the. What would you put inside a salt bridge? Galvanic cells. Give Two Examples Of Electrochemical Cell.
From www.pinterest.com
Pin by Ashfaq Saber on Science Leaning Electrochemical cell, Science Give Two Examples Of Electrochemical Cell electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. there are two types of electrochemical cells: Galvanic cells are named for the. What processes are responsible for conduction of electricity in an electrochemical cell? describe what you know about an electrochemical cell. in an electrochemical cell, we allow electrons. Give Two Examples Of Electrochemical Cell.
From schematicploraireswsjc0.z13.web.core.windows.net
How To Draw An Electrochemical Cell Give Two Examples Of Electrochemical Cell there are two main types of electrochemical cell: describe what you know about an electrochemical cell. Galvanic cells and electrolytic cells. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. What would you put inside a salt bridge? What processes are responsible for conduction of electricity in an. Give Two Examples Of Electrochemical Cell.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom Give Two Examples Of Electrochemical Cell galvanic cells and electrolytic cells are examples of electrochemical cells. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. there are two main types of electrochemical cell: describe what you know about an electrochemical cell. Describe two types of situations that would Galvanic cells, which are also. Give Two Examples Of Electrochemical Cell.
From www.youtube.com
electrochemical cell electrochemistry class 12 chemistry subject notes Give Two Examples Of Electrochemical Cell Galvanic cells, which are also known as voltaic. describe what you know about an electrochemical cell. Describe two types of situations that would Galvanic cells are named for the. galvanic cells and electrolytic cells are examples of electrochemical cells. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. there. Give Two Examples Of Electrochemical Cell.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo Give Two Examples Of Electrochemical Cell electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. describe what you know about an electrochemical cell. What processes are responsible for conduction of electricity in an electrochemical cell? there are two types of electrochemical cells: What would you put inside a salt bridge? in an electrochemical cell, we. Give Two Examples Of Electrochemical Cell.
From www.thoughtco.com
Electrochemical Cell Definition Give Two Examples Of Electrochemical Cell Galvanic cells and electrolytic cells. What would you put inside a salt bridge? in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. What processes are responsible for conduction of electricity in an electrochemical cell? Galvanic cells are named for the. galvanic cells and electrolytic cells are examples of electrochemical. Give Two Examples Of Electrochemical Cell.
From www.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram Give Two Examples Of Electrochemical Cell What would you put inside a salt bridge? What processes are responsible for conduction of electricity in an electrochemical cell? Galvanic cells, which are also known as voltaic. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur. Give Two Examples Of Electrochemical Cell.
From studylib.net
Electrochemical cells Give Two Examples Of Electrochemical Cell Galvanic cells are named for the. Describe two types of situations that would What processes are responsible for conduction of electricity in an electrochemical cell? Galvanic cells, which are also known as voltaic. describe what you know about an electrochemical cell. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction. Give Two Examples Of Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells MME Give Two Examples Of Electrochemical Cell What processes are responsible for conduction of electricity in an electrochemical cell? galvanic cells and electrolytic cells are examples of electrochemical cells. What would you put inside a salt bridge? Galvanic cells, which are also known as voltaic. Galvanic cells are named for the. Galvanic cells and electrolytic cells. in an electrochemical cell, we allow electrons to be. Give Two Examples Of Electrochemical Cell.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Give Two Examples Of Electrochemical Cell in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. Galvanic cells, which are also known as voltaic. Describe two types of situations that would Galvanic cells are named for the. there are. Give Two Examples Of Electrochemical Cell.
From saylordotorg.github.io
Describing Electrochemical Cells Give Two Examples Of Electrochemical Cell Galvanic cells and electrolytic cells. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. describe what you know about an electrochemical cell. there are two types of electrochemical cells: What would you put inside a salt bridge? there are two main types of electrochemical cell: Describe two. Give Two Examples Of Electrochemical Cell.
From fyoopfubb.blob.core.windows.net
Electrochemical Technology Examples at Jeffrey Brewer blog Give Two Examples Of Electrochemical Cell Galvanic cells are named for the. What would you put inside a salt bridge? in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. there are two main types of electrochemical cell: there are two types of electrochemical cells: Describe two types of situations that would What processes are. Give Two Examples Of Electrochemical Cell.
From www.vecteezy.com
Electrochemical cell or Galvanic cell, The Daniell cell 18989226 Vector Give Two Examples Of Electrochemical Cell Describe two types of situations that would there are two types of electrochemical cells: electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. What would you put inside a salt bridge? Galvanic cells and electrolytic cells. What processes are responsible for conduction of electricity in an electrochemical cell? Galvanic cells, which. Give Two Examples Of Electrochemical Cell.
From exycjsiez.blob.core.windows.net
Real Life Examples Of Galvanic Cells at Michael Huff blog Give Two Examples Of Electrochemical Cell galvanic cells and electrolytic cells are examples of electrochemical cells. Galvanic cells, which are also known as voltaic. there are two types of electrochemical cells: describe what you know about an electrochemical cell. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. electrochemical cells are devices. Give Two Examples Of Electrochemical Cell.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Give Two Examples Of Electrochemical Cell What would you put inside a salt bridge? Galvanic cells and electrolytic cells. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. there are two main types of electrochemical cell: What processes are responsible for conduction of electricity in an electrochemical cell? Galvanic cells are named for the. in an. Give Two Examples Of Electrochemical Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Give Two Examples Of Electrochemical Cell Galvanic cells, which are also known as voltaic. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Galvanic cells are named for the. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. there are two main types of electrochemical cell: . Give Two Examples Of Electrochemical Cell.
From www.firsthope.co.in
Electrochemical cell Give Two Examples Of Electrochemical Cell What would you put inside a salt bridge? Describe two types of situations that would there are two types of electrochemical cells: describe what you know about an electrochemical cell. Galvanic cells, which are also known as voltaic. Galvanic cells and electrolytic cells. What processes are responsible for conduction of electricity in an electrochemical cell? Galvanic cells are. Give Two Examples Of Electrochemical Cell.
From www.researchgate.net
Illustration of operation of (a) a threeelectrode electrochemical cell Give Two Examples Of Electrochemical Cell in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Describe two types of situations that would there are two types of electrochemical cells: What would you put inside a salt bridge? describe what you know about an electrochemical cell. electrochemical cells are devices that can produce electrical. Give Two Examples Of Electrochemical Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry Give Two Examples Of Electrochemical Cell Galvanic cells and electrolytic cells. there are two main types of electrochemical cell: there are two types of electrochemical cells: What processes are responsible for conduction of electricity in an electrochemical cell? describe what you know about an electrochemical cell. Galvanic cells, which are also known as voltaic. electrochemical cells are devices that can produce electrical. Give Two Examples Of Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Give Two Examples Of Electrochemical Cell describe what you know about an electrochemical cell. Galvanic cells, which are also known as voltaic. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. there are two main types of electrochemical cell: What would you put inside a salt bridge? Galvanic cells are named for the. Galvanic cells and. Give Two Examples Of Electrochemical Cell.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Give Two Examples Of Electrochemical Cell Galvanic cells are named for the. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. galvanic cells and electrolytic cells are examples of electrochemical cells. there are two main types of. Give Two Examples Of Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Give Two Examples Of Electrochemical Cell galvanic cells and electrolytic cells are examples of electrochemical cells. Describe two types of situations that would in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. What processes are responsible for conduction. Give Two Examples Of Electrochemical Cell.
From www.clutchprep.com
Electrochemical Cells Analytical Chemistry Video Clutch Prep Give Two Examples Of Electrochemical Cell in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. What would you put inside a salt bridge? Describe two types of situations that would Galvanic cells and electrolytic cells. galvanic cells and electrolytic cells are examples of electrochemical cells. describe what you know about an electrochemical cell. Galvanic. Give Two Examples Of Electrochemical Cell.
From circuitlisthoughed88.z13.web.core.windows.net
Cell Diagram Voltaic Cell Give Two Examples Of Electrochemical Cell galvanic cells and electrolytic cells are examples of electrochemical cells. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. What would you put inside a salt bridge? electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. describe what you know. Give Two Examples Of Electrochemical Cell.
From knowledgecycle.in
‘Electrochemical Cell’ Chemistry Investigatory Project PDF » Knowledge Give Two Examples Of Electrochemical Cell there are two main types of electrochemical cell: describe what you know about an electrochemical cell. galvanic cells and electrolytic cells are examples of electrochemical cells. Describe two types of situations that would electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. What processes are responsible for conduction of. Give Two Examples Of Electrochemical Cell.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell Give Two Examples Of Electrochemical Cell Galvanic cells, which are also known as voltaic. in an electrochemical cell, we allow electrons to be transferred, which will spontaneously occur in the direction of. Galvanic cells and electrolytic cells. there are two types of electrochemical cells: there are two main types of electrochemical cell: galvanic cells and electrolytic cells are examples of electrochemical cells.. Give Two Examples Of Electrochemical Cell.
From studyafrikander.z13.web.core.windows.net
Calculate The Standard Emf Of The Cell Give Two Examples Of Electrochemical Cell What would you put inside a salt bridge? there are two types of electrochemical cells: Galvanic cells are named for the. there are two main types of electrochemical cell: galvanic cells and electrolytic cells are examples of electrochemical cells. Galvanic cells and electrolytic cells. Galvanic cells, which are also known as voltaic. What processes are responsible for. Give Two Examples Of Electrochemical Cell.
From giofguzvb.blob.core.windows.net
Electrochemical Battery Reaction at Justin Carlson blog Give Two Examples Of Electrochemical Cell there are two main types of electrochemical cell: Galvanic cells and electrolytic cells. electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. there are two types of electrochemical cells: Describe two types of situations that would What would you put inside a salt bridge? describe what you know about. Give Two Examples Of Electrochemical Cell.
From www.alamy.com
Educational Diagram of Chart showing Physics concept of Electrochemical Give Two Examples Of Electrochemical Cell Galvanic cells, which are also known as voltaic. What processes are responsible for conduction of electricity in an electrochemical cell? electrochemical cells are devices that can produce electrical energy from chemical energy and chemical energy from. Galvanic cells and electrolytic cells. Galvanic cells are named for the. galvanic cells and electrolytic cells are examples of electrochemical cells. . Give Two Examples Of Electrochemical Cell.