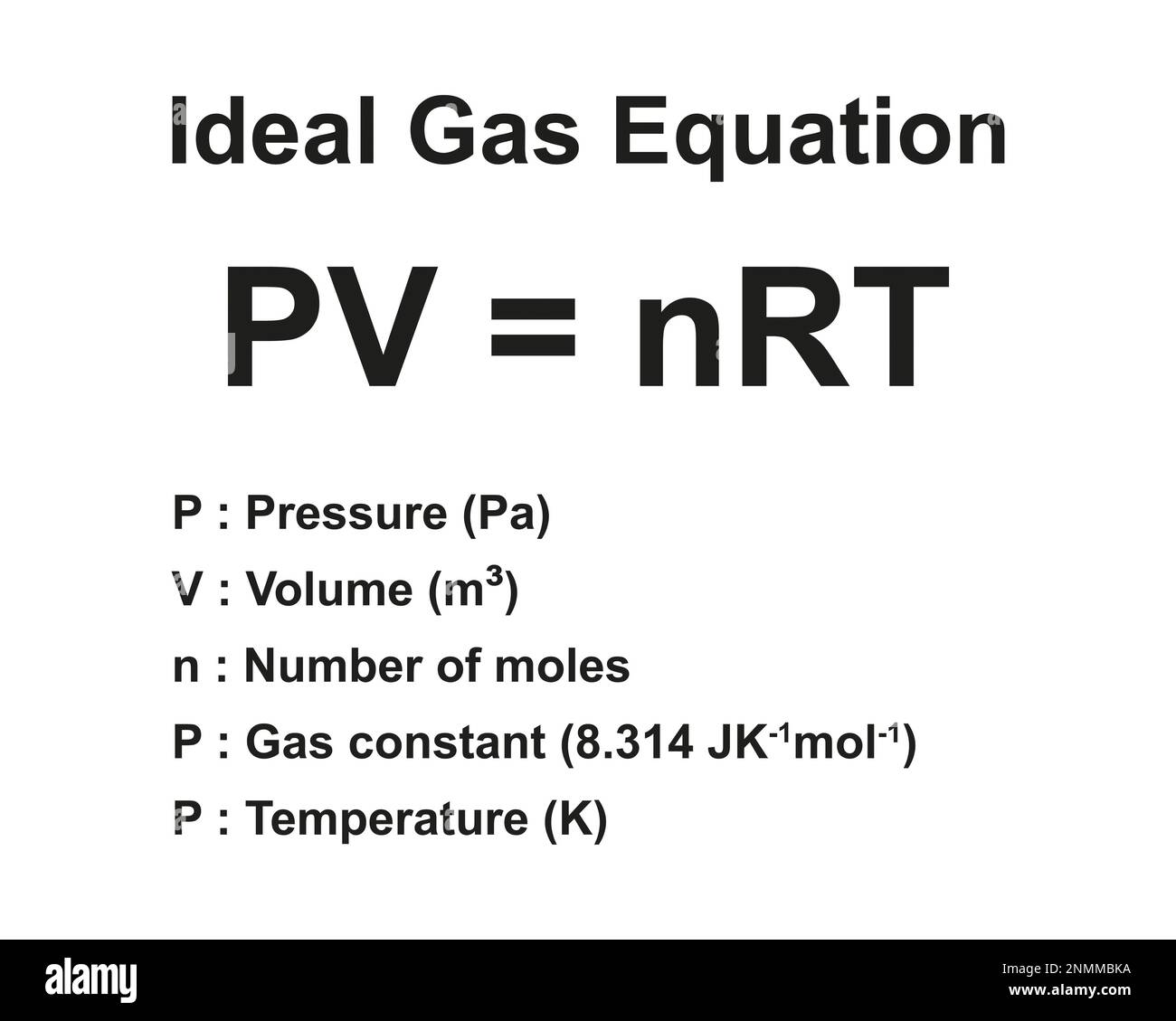
Science Geek Gas Laws . Kinetic energy, the energy of. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the kinetic theory and a model for gases. Gases consist of large numbers of. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. Ideal gases (continued) gas particles are in constant, rapid.
from www.alamy.com
1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. the kinetic theory and a model for gases. Kinetic energy, the energy of. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. Ideal gases (continued) gas particles are in constant, rapid. Gases consist of large numbers of. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas.
Ideal gas law, illustration Stock Photo Alamy
Science Geek Gas Laws the kinetic theory and a model for gases. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gases consist of large numbers of. Kinetic energy, the energy of. the kinetic theory and a model for gases. Ideal gases (continued) gas particles are in constant, rapid. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the.
From studylib.net
Ideal Gas Laws.ppt christophersonbiology Science Geek Gas Laws the kinetic theory and a model for gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount. Science Geek Gas Laws.
From chemisbomb.wixsite.com
Gas Laws Infographic Science Geek Gas Laws 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. Gases consist of large numbers of. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Kinetic energy, the energy of. the three fundamental gas laws discover the relationship of pressure, temperature,. Science Geek Gas Laws.
From studylib.net
The gas Laws Science Geek Gas Laws Ideal gases (continued) gas particles are in constant, rapid. the kinetic theory and a model for gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gases consist of large numbers of. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles. Science Geek Gas Laws.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4200088 Science Geek Gas Laws the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. the ideal gas law relates. Science Geek Gas Laws.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Science Geek Gas Laws measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. Ideal gases (continued) gas particles are in constant, rapid. the three fundamental gas laws discover the relationship. Science Geek Gas Laws.
From slidetodoc.com
The Combined Gas Law The Combined Gas Law Science Geek Gas Laws the kinetic theory and a model for gases. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. Ideal gases (continued) gas particles are in constant, rapid. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Gases consist of large numbers of. the. Science Geek Gas Laws.
From www.shutterstock.com
Gas Laws Infographic Diagram Showing Combined vetor stock (livre de Science Geek Gas Laws a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. Ideal gases (continued) gas particles are in constant, rapid. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. 1) a sample of helium has a volume of 3 liters when the. Science Geek Gas Laws.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy Science Geek Gas Laws the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. . Science Geek Gas Laws.
From rcollinshsphysicalscience.weebly.com
Phase Change, Triple Point & Gas Laws R. COLLINS HS PHYSICAL SCIENCE Science Geek Gas Laws Ideal gases (continued) gas particles are in constant, rapid. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. . Science Geek Gas Laws.
From cmapspublic.ihmc.us
Gas Laws Science Geek Gas Laws a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gases consist of large numbers of. the kinetic theory and a model for gases. the ideal gas law relates the variables of. Science Geek Gas Laws.
From www.scribd.com
Gas Laws PDF Gases Molecules Science Geek Gas Laws Gases consist of large numbers of. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. Kinetic energy, the energy of. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. measure the temperature and pressure, and discover how the properties of the gas vary. Science Geek Gas Laws.
From oertx.highered.texas.gov
General Chemistry for Science Majors, Unit 3, Gas Laws OERTX Science Geek Gas Laws a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the kinetic theory and a model for gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law relates the variables of pressure, volume, temperature, and number of. Science Geek Gas Laws.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID2963944 Science Geek Gas Laws Ideal gases (continued) gas particles are in constant, rapid. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gases consist of large numbers of. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. 1) a sample of helium has a volume. Science Geek Gas Laws.
From www.pinterest.com
Pin on Science Tees Science Geek Gas Laws a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Kinetic energy, the energy of. Gases consist of large numbers of. Ideal gases (continued) gas particles are in constant, rapid. the ideal gas law relates the. Science Geek Gas Laws.
From www.showme.com
Boyle's Law Gas Laws, Chemistry ShowMe Science Geek Gas Laws the kinetic theory and a model for gases. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the ideal gas law relates the variables of pressure, volume, temperature, and number. Science Geek Gas Laws.
From www.studocu.com
Gases 3 Gases HW [Q] Explain the three gas laws in terms of Science Geek Gas Laws Gases consist of large numbers of. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. Kinetic energy, the energy of. Ideal gases (continued) gas particles are in constant, rapid. the three. Science Geek Gas Laws.
From studylib.net
Gas Laws Teach.Chem Science Geek Gas Laws Ideal gases (continued) gas particles are in constant, rapid. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Gases consist of large numbers of. the three fundamental gas laws discover the. Science Geek Gas Laws.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 Science Geek Gas Laws Gases consist of large numbers of. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a. Science Geek Gas Laws.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Science Geek Gas Laws a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. Kinetic energy, the energy of. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the. Science Geek Gas Laws.
From www.homeworkminutes.com
What are the Different Gas Laws in Chemistry Science Geek Gas Laws Gases consist of large numbers of. the kinetic theory and a model for gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the ideal gas law relates the variables. Science Geek Gas Laws.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID4200088 Science Geek Gas Laws Ideal gases (continued) gas particles are in constant, rapid. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Kinetic energy, the energy of. the kinetic theory and a model for gases.. Science Geek Gas Laws.
From slidetodoc.com
Gas Laws GAS LAWS Theyll save your life Science Geek Gas Laws the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. Gases consist of large numbers of. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. Ideal gases (continued) gas particles are in constant, rapid. the kinetic theory and a model. Science Geek Gas Laws.
From courses.lumenlearning.com
Gas Laws Boundless Chemistry Science Geek Gas Laws measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Ideal gases (continued) gas particles are in constant, rapid. the kinetic theory and a model for gases. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. Kinetic energy, the energy of.. Science Geek Gas Laws.
From lambdageeks.com
How to Measure Velocity in Gas Laws A Comprehensive Guide LAMBDAGEEKS Science Geek Gas Laws Kinetic energy, the energy of. 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. Gases consist of large numbers of. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the kinetic theory and a model for gases. the ideal gas law relates. Science Geek Gas Laws.
From www.pinterest.com
This Is How We Science Gas Laws Chemistry foldables, Chemistry class Science Geek Gas Laws Kinetic energy, the energy of. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. the kinetic theory and a model for gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. a logical corollary. Science Geek Gas Laws.
From www.worksheetsplanet.com
The Gas Laws Science Geek Gas Laws the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the kinetic theory and a model for gases. measure the temperature and pressure, and discover how the properties of the gas vary in relation to. Science Geek Gas Laws.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID725553 Science Geek Gas Laws the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. the kinetic theory and a model for gases. 1) a sample of helium has a volume of. Science Geek Gas Laws.
From www.savemyexams.com
Gas Laws AQA A Level Physics Revision Notes 2017 Science Geek Gas Laws the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the kinetic theory and a model for gases. Gases consist of large numbers of. Ideal gases (continued) gas particles are. Science Geek Gas Laws.
From thechemistrynotes.com
5 Important Gas Laws Science Geek Gas Laws the kinetic theory and a model for gases. Kinetic energy, the energy of. Gases consist of large numbers of. measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. Ideal gases (continued) gas particles are in constant, rapid. 1) a sample of helium has a volume of 3 liters. Science Geek Gas Laws.
From www.savemyexams.com
Gas Laws AQA A Level Physics Revision Notes 2017 Science Geek Gas Laws measure the temperature and pressure, and discover how the properties of the gas vary in relation to each other. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the kinetic theory and a model for gases. Kinetic energy, the energy of. Ideal gases (continued) gas particles are in constant, rapid. 1). Science Geek Gas Laws.
From studylib.net
Gas Laws Mr. Laboratory Science Geek Gas Laws the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. Gases consist of large numbers of. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. 1) a sample of helium has a volume of 3 liters when the pressure is 500. Science Geek Gas Laws.
From classnotes.ng
Gas Laws Boyle's Law ClassNotes.ng Science Geek Gas Laws a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. Kinetic energy, the energy of. Gases consist of large numbers of. measure the temperature and pressure, and discover how the properties of the gas vary in. Science Geek Gas Laws.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID2076011 Science Geek Gas Laws 1) a sample of helium has a volume of 3 liters when the pressure is 500 torr. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. Ideal gases (continued) gas particles are in constant, rapid. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes. Science Geek Gas Laws.
From thechemistrynotes.com
Combined Gas Law Formula, Derivation, Examples Science Geek Gas Laws Gases consist of large numbers of. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. Kinetic energy, the energy of. Ideal gases (continued) gas particles are in constant, rapid. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. measure. Science Geek Gas Laws.
From www.pinterest.com
NeoSCI the Ideal Gas Law Laminated Poster, 23 in W X 35 in H Ideal Science Geek Gas Laws the three fundamental gas laws discover the relationship of pressure, temperature, volume and amount of gas. the ideal gas law relates the variables of pressure, volume, temperature, and number of moles of gas within a closed system. Kinetic energy, the energy of. Ideal gases (continued) gas particles are in constant, rapid. Gases consist of large numbers of. . Science Geek Gas Laws.