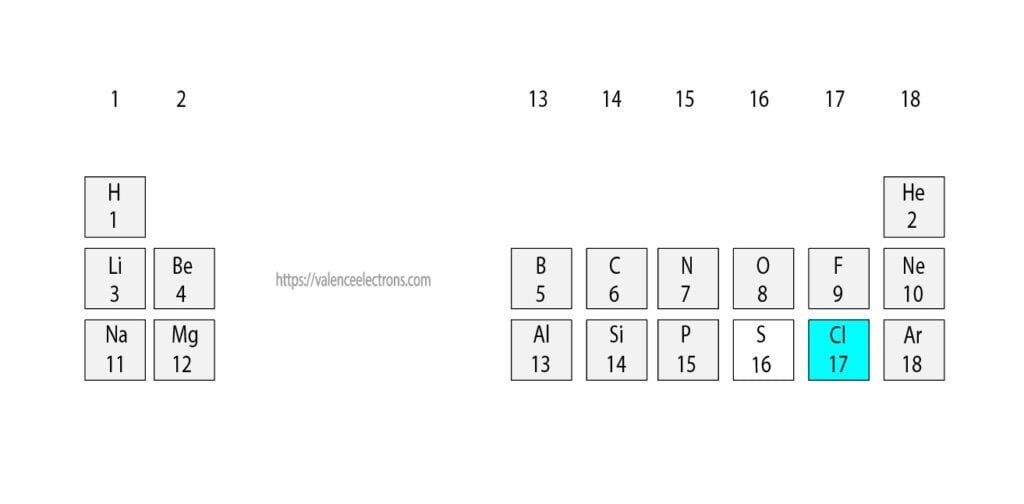
Chlorine Total Valence Electrons . Each h atom (group 1) has 1 valence electron, and the. Calcium would have two valence electrons, since it is in group iia (group 2). Thus, chlorine has seven valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). The 10 remaining electrons, from the first and second shells, are core electrons. Determine the total number of valence electrons in the molecule or ion. The first energy level holds a maximum of two valence electrons. That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Examples of to use our electron configuration calculator faqs. Helium is the only exception for the main group elements.
from dekookguide.com
The 10 remaining electrons, from the first and second shells, are core electrons. Examples of to use our electron configuration calculator faqs. That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. Helium is the only exception for the main group elements. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Determine the total number of valence electrons in the molecule or ion. Calcium would have two valence electrons, since it is in group iia (group 2). There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). The first energy level holds a maximum of two valence electrons.
How Many Valence Electrons Does Chlorine Have DeKookGuide
Chlorine Total Valence Electrons Each h atom (group 1) has 1 valence electron, and the. Helium is the only exception for the main group elements. Each h atom (group 1) has 1 valence electron, and the. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Determine the total number of valence electrons in the molecule or ion. Calcium would have two valence electrons, since it is in group iia (group 2). Examples of to use our electron configuration calculator faqs. Thus, chlorine has seven valence electrons. The first energy level holds a maximum of two valence electrons. That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. The 10 remaining electrons, from the first and second shells, are core electrons.
From favpng.com
Electron Configuration Aufbau Principle Valence Electron Chlorine, PNG Chlorine Total Valence Electrons That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Thus, chlorine has seven valence electrons. Each h atom (group 1) has 1 valence electron, and the. The first energy level holds a maximum of two valence electrons. Helium is the only exception for the main group elements. Determine the total number of valence electrons. Chlorine Total Valence Electrons.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Chlorine Total Valence Electrons That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Each h atom (group 1) has 1 valence electron, and the. The 10 remaining electrons, from the first and second shells, are core electrons. Helium is the only exception for the main group elements. 93 rows you may assume the valences of the chemical elements—the. Chlorine Total Valence Electrons.
From www.slideserve.com
PPT Electron Energy Levels and Configurations PowerPoint Presentation Chlorine Total Valence Electrons That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Determine the total number of valence electrons in the molecule or ion. Examples of to use our electron configuration calculator faqs. Chlorine is in group viia (group 17), so it would have seven valence electrons. There are a total of seven electrons present in the. Chlorine Total Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Chloromethane? Chlorine Total Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Thus, chlorine has seven valence electrons. The. Chlorine Total Valence Electrons.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Total Valence Electrons Helium is the only exception for the main group elements. The first energy level holds a maximum of two valence electrons. The 10 remaining electrons, from the first and second shells, are core electrons. Each h atom (group 1) has 1 valence electron, and the. Calcium would have two valence electrons, since it is in group iia (group 2). Examples. Chlorine Total Valence Electrons.
From www.chegg.com
Solved What is the electron configuration for Cl, chlorine, Chlorine Total Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). Helium is the only exception for the main group elements. Calcium would have two valence electrons, since it is in. Chlorine Total Valence Electrons.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Total Valence Electrons The first energy level holds a maximum of two valence electrons. Helium is the only exception for the main group elements. The 10 remaining electrons, from the first and second shells, are core electrons. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). Thus, chlorine has seven valence electrons. 93 rows you may. Chlorine Total Valence Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Total Valence Electrons That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Helium is the only exception for the main group elements. Examples of to use our electron configuration calculator faqs. Each h atom (group 1) has 1 valence electron, and the. The first energy level holds a maximum of two valence electrons. The 10 remaining electrons,. Chlorine Total Valence Electrons.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Total Valence Electrons Thus, chlorine has seven valence electrons. Helium is the only exception for the main group elements. Calcium would have two valence electrons, since it is in group iia (group 2). There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). Examples of to use our electron configuration calculator faqs. That gives a total of. Chlorine Total Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Chlorine Total Valence Electrons The first energy level holds a maximum of two valence electrons. That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. The 10 remaining electrons, from the first and second shells, are core electrons. Calcium would have two valence electrons, since. Chlorine Total Valence Electrons.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Total Valence Electrons That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). Calcium would have two valence electrons, since it is in group iia (group 2). Examples. Chlorine Total Valence Electrons.
From www.ck12.org
Valence Electrons CK12 Foundation Chlorine Total Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Examples of to use our electron configuration calculator faqs. The first energy level holds a maximum of two valence electrons. Thus, chlorine has seven valence electrons. Each h atom (group 1) has 1 valence electron, and. Chlorine Total Valence Electrons.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Total Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine is in group viia (group 17), so it would have seven valence electrons. Thus, chlorine has seven valence electrons. That gives. Chlorine Total Valence Electrons.
From dekookguide.com
How Many Valence Electrons Does Chlorine Have DeKookGuide Chlorine Total Valence Electrons There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). Calcium would have two valence electrons, since it is in group iia (group 2). Determine the total number of valence electrons in the molecule or ion. Helium is the only exception for the main group elements. Each h atom (group 1) has 1 valence. Chlorine Total Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for PCl3? Chlorine Total Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). The 10 remaining electrons, from the first and second shells, are core electrons. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). Helium is the only exception for the main group elements. Examples of to use our electron configuration. Chlorine Total Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does ClO2 and ClO2 Have? Chlorine Total Valence Electrons Each h atom (group 1) has 1 valence electron, and the. Thus, chlorine has seven valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). Determine the total number of valence electrons in the molecule or ion. Helium is the. Chlorine Total Valence Electrons.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Total Valence Electrons That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. Thus, chlorine has seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Calcium. Chlorine Total Valence Electrons.
From brokeasshome.com
Periodic Table Chlorine Electrons Chlorine Total Valence Electrons Each h atom (group 1) has 1 valence electron, and the. The first energy level holds a maximum of two valence electrons. Determine the total number of valence electrons in the molecule or ion. Calcium would have two valence electrons, since it is in group iia (group 2). 93 rows you may assume the valences of the chemical elements—the number. Chlorine Total Valence Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Total Valence Electrons Chlorine is in group viia (group 17), so it would have seven valence electrons. Examples of to use our electron configuration calculator faqs. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The first energy level holds a maximum of two valence electrons. Helium is. Chlorine Total Valence Electrons.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Total Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). Chlorine is in group viia (group 17), so it would have seven valence electrons. Thus, chlorine has seven valence electrons. Helium is the only exception for the main group elements. The first energy level holds a maximum of two valence electrons. Examples of to use our. Chlorine Total Valence Electrons.
From www.slideserve.com
PPT Objective 4 PowerPoint Presentation, free download ID1886947 Chlorine Total Valence Electrons Each h atom (group 1) has 1 valence electron, and the. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine is in group viia (group 17), so it. Chlorine Total Valence Electrons.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Total Valence Electrons The 10 remaining electrons, from the first and second shells, are core electrons. Examples of to use our electron configuration calculator faqs. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine is in group viia (group 17), so it would have seven valence electrons.. Chlorine Total Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Chlorine Total Valence Electrons The 10 remaining electrons, from the first and second shells, are core electrons. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). The first energy level holds a maximum of two valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). Examples of to use our electron. Chlorine Total Valence Electrons.
From favpng.com
Lewis Structure Chlorine Valence Electron Diagram, PNG, 2000x2000px Chlorine Total Valence Electrons Thus, chlorine has seven valence electrons. Each h atom (group 1) has 1 valence electron, and the. Helium is the only exception for the main group elements. Examples of to use our electron configuration calculator faqs. Calcium would have two valence electrons, since it is in group iia (group 2). 93 rows you may assume the valences of the chemical. Chlorine Total Valence Electrons.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Total Valence Electrons That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Each h atom (group 1) has 1 valence electron, and the. Helium is the only exception for the main group elements. Determine the total number of valence electrons in the molecule or ion. Thus, chlorine has seven valence electrons. The 10 remaining electrons, from the. Chlorine Total Valence Electrons.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Total Valence Electrons That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Examples of to use our electron configuration calculator faqs. Helium is the only exception for the main group elements. The first energy level holds a maximum of two valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). Determine. Chlorine Total Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Chlorine (Cl)? Chlorine Total Valence Electrons Helium is the only exception for the main group elements. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Chlorine is in group viia (group 17), so it would have. Chlorine Total Valence Electrons.
From byjus.com
Find out the number of valence electrons present in chlorine. Chlorine Total Valence Electrons Examples of to use our electron configuration calculator faqs. Thus, chlorine has seven valence electrons. That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Determine the total number of valence electrons in the molecule or ion. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Chlorine Total Valence Electrons.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Chlorine Total Valence Electrons The first energy level holds a maximum of two valence electrons. That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Thus, chlorine has seven valence electrons. There are a total of seven electrons present in the valence shell/outermost shell of chlorine (3s²3p⁵). Chlorine is in group viia (group 17), so it would have seven. Chlorine Total Valence Electrons.
From imgbin.com
Lewis Structure Chlorine Valence Electron Diagram PNG, Clipart, Atom Chlorine Total Valence Electrons Chlorine is in group viia (group 17), so it would have seven valence electrons. Thus, chlorine has seven valence electrons. The first energy level holds a maximum of two valence electrons. Examples of to use our electron configuration calculator faqs. The 10 remaining electrons, from the first and second shells, are core electrons. Helium is the only exception for the. Chlorine Total Valence Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Total Valence Electrons Thus, chlorine has seven valence electrons. Helium is the only exception for the main group elements. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Calcium would have two valence electrons, since it is in group iia (group 2). Examples of to use our electron. Chlorine Total Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for COCl2 (phosgene)? Chlorine Total Valence Electrons The first energy level holds a maximum of two valence electrons. That gives a total of 7 electrons, so neutral chlorine atoms have 7 valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). Helium is the only exception for the main group elements. The 10 remaining electrons, from the first and second shells,. Chlorine Total Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Total Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). The first energy level holds a maximum of two valence electrons. Each h atom (group 1) has 1 valence electron, and the. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Chlorine Total Valence Electrons.
From www.chemtopper.com
How to draw Lewis Dot Structure Online Chemistry Tutor Chlorine Total Valence Electrons Helium is the only exception for the main group elements. Chlorine is in group viia (group 17), so it would have seven valence electrons. Determine the total number of valence electrons in the molecule or ion. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Chlorine Total Valence Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Total Valence Electrons Thus, chlorine has seven valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). Examples of to use our electron configuration calculator faqs. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an. Chlorine Total Valence Electrons.