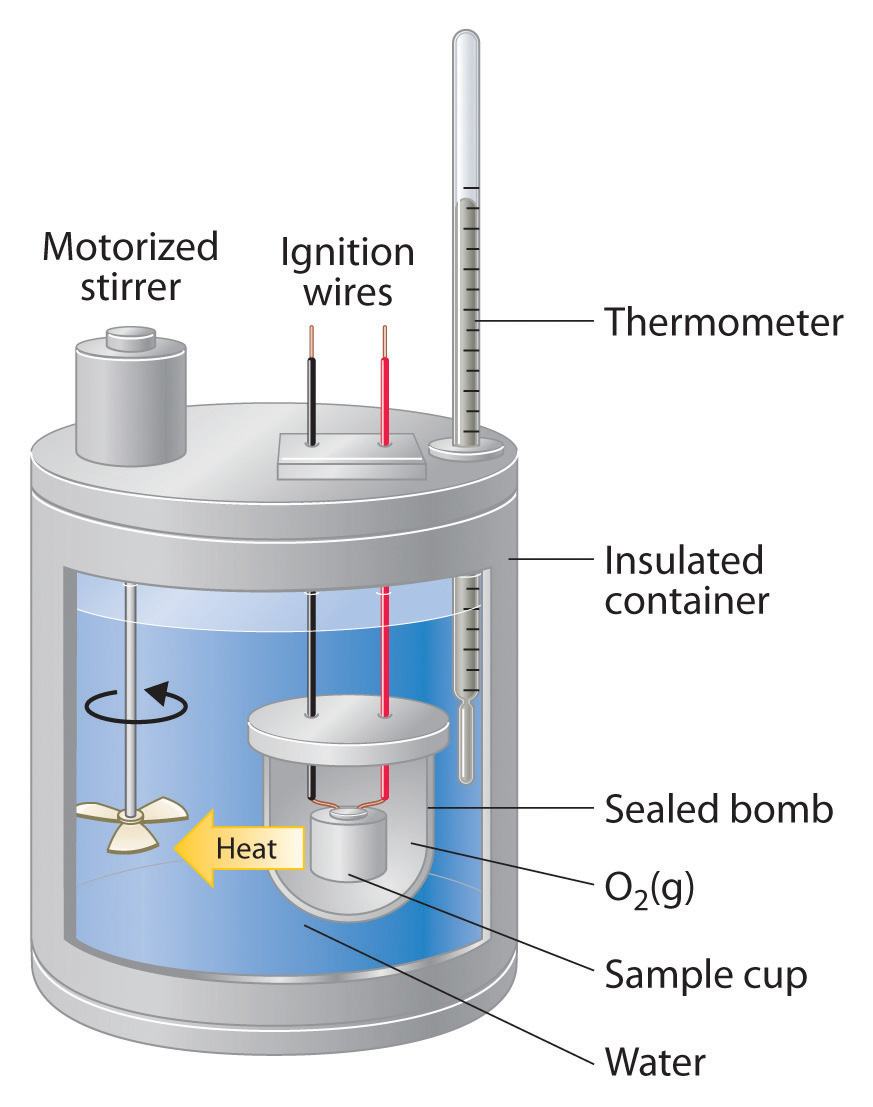
How Do Calorimeters Measure Heat Energy . Calorimetry is used to measure amounts of heat transferred to. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid.
from saylordotorg.github.io
Apply the first law of thermodynamics to calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and.
Calorimetry
How Do Calorimeters Measure Heat Energy Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. To do so, the heat is exchanged with a calibrated object. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry.
From saylordotorg.github.io
Calorimetry How Do Calorimeters Measure Heat Energy A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. How Do Calorimeters Measure Heat Energy.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube How Do Calorimeters Measure Heat Energy Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot to. How Do Calorimeters Measure Heat Energy.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow How Do Calorimeters Measure Heat Energy Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to. To do so, the heat is exchanged with a calibrated object. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount. How Do Calorimeters Measure Heat Energy.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] How Do Calorimeters Measure Heat Energy A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter. How Do Calorimeters Measure Heat Energy.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Calorimetry is used to measure amounts of heat transferred to or from. How Do Calorimeters Measure Heat Energy.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Do Calorimeters Measure Heat Energy Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. How Do Calorimeters Measure Heat Energy.
From thermonine92.blogspot.com
Thermochemistry Calorimeter How Do Calorimeters Measure Heat Energy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. To do so, the. How Do Calorimeters Measure Heat Energy.
From users.highland.edu
Calorimetry How Do Calorimeters Measure Heat Energy Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in. How Do Calorimeters Measure Heat Energy.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry measures enthalpy changes during chemical processes, where the. How Do Calorimeters Measure Heat Energy.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec How Do Calorimeters Measure Heat Energy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 How Do Calorimeters Measure Heat Energy Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. To do so, the heat is. How Do Calorimeters Measure Heat Energy.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 How Do Calorimeters Measure Heat Energy To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is used to measure amounts of heat transferred to. A calorimeter measures the mass of liquid and the temperature. How Do Calorimeters Measure Heat Energy.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. Calorimetry. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID6317093 How Do Calorimeters Measure Heat Energy Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity. How Do Calorimeters Measure Heat Energy.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object. Calorimetry is used to measure amounts of heat transferred to. Apply the first law of thermodynamics to calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the. How Do Calorimeters Measure Heat Energy.
From slideplayer.com
Chapter 2 Matter and Energy ppt download How Do Calorimeters Measure Heat Energy Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6133898 How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and.. How Do Calorimeters Measure Heat Energy.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. How Do Calorimeters Measure Heat Energy.
From www.youtube.com
Principle of Calorimetry YouTube How Do Calorimeters Measure Heat Energy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry is used to measure amounts of heat transferred to or from a substance. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to. To do so, the. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT A calorimeter is used to measure the amount of heat absorbed or How Do Calorimeters Measure Heat Energy Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object. Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT Chapter 2 Energy and Matter PowerPoint Presentation, free How Do Calorimeters Measure Heat Energy Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT Thermal Energy and Matter PowerPoint Presentation, free download How Do Calorimeters Measure Heat Energy Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure. How Do Calorimeters Measure Heat Energy.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts How Do Calorimeters Measure Heat Energy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained. How Do Calorimeters Measure Heat Energy.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry How Do Calorimeters Measure Heat Energy A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Apply the first law of thermodynamics to calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry is. How Do Calorimeters Measure Heat Energy.
From studylib.net
Chapter 5b (PowerPoint) How Do Calorimeters Measure Heat Energy To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the. How Do Calorimeters Measure Heat Energy.
From studylib.net
Heat Equation How Do Calorimeters Measure Heat Energy A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. To do so, the heat. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download How Do Calorimeters Measure Heat Energy A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. One technique we can use to measure the amount of heat. How Do Calorimeters Measure Heat Energy.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download How Do Calorimeters Measure Heat Energy A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry is used to measure amounts of heat transferred to. How Do Calorimeters Measure Heat Energy.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation How Do Calorimeters Measure Heat Energy Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used to measure amounts of heat transferred to or from a substance. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. To do so, the heat is exchanged with a calibrated. How Do Calorimeters Measure Heat Energy.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube How Do Calorimeters Measure Heat Energy Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is used. How Do Calorimeters Measure Heat Energy.
From www.wikihow.com
How to Measure Thermal Energy Released from a Flame 10 Steps How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter measures the mass of liquid and the temperature change of the liquid to determine the quantity of energy gained or lost by the liquid. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. How Do Calorimeters Measure Heat Energy.
From courses.lumenlearning.com
Calorimetry General Chemistry How Do Calorimeters Measure Heat Energy Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter measures the. How Do Calorimeters Measure Heat Energy.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How Do Calorimeters Measure Heat Energy Calorimetry is used to measure amounts of heat transferred to. To do so, the heat is exchanged with a calibrated object. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of heat released or absorbed and. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real.. How Do Calorimeters Measure Heat Energy.
From slideplayer.com
Chapter 2 Energy and Matter ppt download How Do Calorimeters Measure Heat Energy One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Apply the first law of thermodynamics to calorimetry. Calorimetry is used to measure amounts of heat transferred to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. How Do Calorimeters Measure Heat Energy.