Solubility Lab Sources Of Error . Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. So, what are the particular sources of each error? By the end of this lab, students should be able to: Set up an experimental work station to. Physical and chemical laboratory experiments include three primary sources of error: Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. Simplify by grouping sources, quantify sources and convert components to standard uncertainties. Properly use an analytical balance to measure mass. Systematic error, random error and human error. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. These sources of errors in lab should be studied well before any further action.
from www.chegg.com
By the end of this lab, students should be able to: So, what are the particular sources of each error? One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Properly use an analytical balance to measure mass. These sources of errors in lab should be studied well before any further action. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. Systematic error, random error and human error. Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. Physical and chemical laboratory experiments include three primary sources of error:
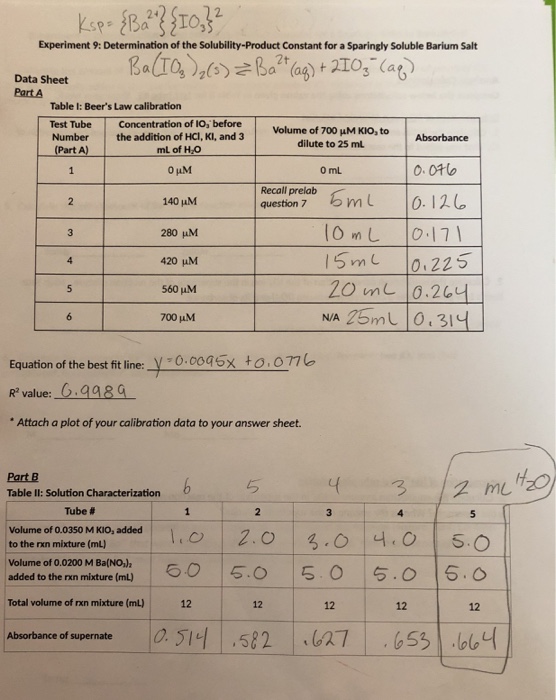
Solved Experiment 9 Determination of the SolubilityProduct
Solubility Lab Sources Of Error The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. So, what are the particular sources of each error? Properly use an analytical balance to measure mass. By the end of this lab, students should be able to: These sources of errors in lab should be studied well before any further action. Set up an experimental work station to. Physical and chemical laboratory experiments include three primary sources of error: Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Simplify by grouping sources, quantify sources and convert components to standard uncertainties. Systematic error, random error and human error.
From www.studocu.com
Lab 11B lab 11b to review solubility Lab11B Problem Solving Solubility Lab Sources Of Error Systematic error, random error and human error. Properly use an analytical balance to measure mass. Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. The simplest. Solubility Lab Sources Of Error.
From www.numerade.com
⏩SOLVEDCalculate the relative error in solubility by using… Numerade Solubility Lab Sources Of Error The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. So, what are the particular sources. Solubility Lab Sources Of Error.
From www.studypool.com
SOLUTION Solubility test of organic compounds experiment Studypool Solubility Lab Sources Of Error Properly use an analytical balance to measure mass. Physical and chemical laboratory experiments include three primary sources of error: Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. So,. Solubility Lab Sources Of Error.
From www.scribd.com
lab solubility and temperature Solubility Solution Solubility Lab Sources Of Error Set up an experimental work station to. Simplify by grouping sources, quantify sources and convert components to standard uncertainties. Properly use an analytical balance to measure mass. By the end of this lab, students should be able to: So, what are the particular sources of each error? Systematic error, random error and human error. Several factors can cause errors in. Solubility Lab Sources Of Error.
From www.chegg.com
Solved Calculate the \ relative error in solubility by Solubility Lab Sources Of Error Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. Physical and chemical laboratory experiments include three primary sources of error: The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Properly use an analytical. Solubility Lab Sources Of Error.
From www.studypool.com
SOLUTION Factors affecting solubility activity experiment sheet Solubility Lab Sources Of Error Systematic error, random error and human error. So, what are the particular sources of each error? The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Physical and chemical laboratory experiments include three primary sources of error: Simplify by grouping sources, quantify. Solubility Lab Sources Of Error.
From www.studocu.com
Solubility Lab Report Lecture notes 1,37 Solubility Lab Report Solubility Lab Sources Of Error The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Systematic error, random error and human error. These sources. Solubility Lab Sources Of Error.
From www.chegg.com
Calculate the relative error in solubility by using Solubility Lab Sources Of Error So, what are the particular sources of each error? Systematic error, random error and human error. Set up an experimental work station to. Physical and chemical laboratory experiments include three primary sources of error: Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical.. Solubility Lab Sources Of Error.
From www.chegg.com
Solved SOLUBILITY AND SPONTANEITY In an experiment analogous Solubility Lab Sources Of Error The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. By the end of this lab,. Solubility Lab Sources Of Error.
From www.expii.com
Types of Error — Overview & Comparison Expii Solubility Lab Sources Of Error These sources of errors in lab should be studied well before any further action. Set up an experimental work station to. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. Systematic error, random error and human error. Several factors can cause errors in titration findings, including. Solubility Lab Sources Of Error.
From solutionpharmacy.in
Factors Influencing Solubility Solution Parmacy Solubility Lab Sources Of Error Simplify by grouping sources, quantify sources and convert components to standard uncertainties. Systematic error, random error and human error. These sources of errors in lab should be studied well before any further action. Properly use an analytical balance to measure mass. By the end of this lab, students should be able to: Set up an experimental work station to. Several. Solubility Lab Sources Of Error.
From studylib.net
Solubility Lab Teacher`s Notes.doc Solubility Lab Sources Of Error Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Titration is a sensitive analytical method that lets you determine an unknown concentration of a. Solubility Lab Sources Of Error.
From www.studocu.com
Solubility LAB Report Solubility The purpose of this experiment is to Solubility Lab Sources Of Error Set up an experimental work station to. Physical and chemical laboratory experiments include three primary sources of error: Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. By the end of this lab, students should be able to: These sources of errors in lab should be studied well before any further action.. Solubility Lab Sources Of Error.
From studylib.net
Solubility Lab Solubility Lab Sources Of Error Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Several factors can cause errors in. Solubility Lab Sources Of Error.
From studylib.net
Experiment Solubility Rules and Net Ionic Equations Solubility Lab Sources Of Error So, what are the particular sources of each error? Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. Properly use an analytical balance to measure mass. Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical.. Solubility Lab Sources Of Error.
From www.youtube.com
Solubility Lab Updated YouTube Solubility Lab Sources Of Error Physical and chemical laboratory experiments include three primary sources of error: Systematic error, random error and human error. Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a. Solubility Lab Sources Of Error.
From www.chegg.com
Solved Double Replacement Reactions and Solubility PostLab Solubility Lab Sources Of Error Systematic error, random error and human error. These sources of errors in lab should be studied well before any further action. So, what are the particular sources of each error? Set up an experimental work station to. Simplify by grouping sources, quantify sources and convert components to standard uncertainties. The simplest way to deal with errors and inaccuracy in a. Solubility Lab Sources Of Error.
From studylib.net
Solubility Curve Lab Solubility Lab Sources Of Error Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. By the end of this lab, students should be able to: Titration is a sensitive. Solubility Lab Sources Of Error.
From www.flinnsci.ca
Solubility and Temperature A Solubility Curve—ChemTopic™ Lab Activity Solubility Lab Sources Of Error Properly use an analytical balance to measure mass. Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. These sources of errors in lab should be studied well before any further action. One example of a systematic error could be using a ph meter. Solubility Lab Sources Of Error.
From www.youtube.com
CHEM 100 Solubility Experiment YouTube Solubility Lab Sources Of Error These sources of errors in lab should be studied well before any further action. Set up an experimental work station to. Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. Physical and chemical laboratory experiments include three primary sources of error: The solubility of a constituent is reliant on the temperature of. Solubility Lab Sources Of Error.
From mfcheappillstqi.blogspot.com
Solubility Tempterature Lab Gizmo Temperature and Solubility Lab Solubility Lab Sources Of Error Simplify by grouping sources, quantify sources and convert components to standard uncertainties. Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. Physical and chemical laboratory experiments include three primary. Solubility Lab Sources Of Error.
From studylib.net
Unit 1C3 Solubility Curve Lab Solubility Lab Sources Of Error Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. Set up an experimental work station to. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. One example of a systematic error could be using a ph meter that. Solubility Lab Sources Of Error.
From www.studocu.com
Solubility Lab Report Solubility Introduction The purpose of this lab Solubility Lab Sources Of Error Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. So, what are the particular sources of each error? Set up an experimental work station to. These sources of errors in lab should be studied well before any further action. By the end of. Solubility Lab Sources Of Error.
From www.thinkswap.com
Student Experiment Solubility Curve Chemistry Year 11 QCE Thinkswap Solubility Lab Sources Of Error Properly use an analytical balance to measure mass. Systematic error, random error and human error. Physical and chemical laboratory experiments include three primary sources of error: One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. So, what are the particular sources. Solubility Lab Sources Of Error.
From studylib.net
Solubility Lab Solubility Lab Sources Of Error So, what are the particular sources of each error? The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Systematic error, random error and human error. Simplify by grouping sources, quantify sources and convert components to standard uncertainties. These sources of errors. Solubility Lab Sources Of Error.
From studylib.net
Solubility Lab Solubility Lab Sources Of Error One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Simplify by grouping sources, quantify sources and convert components to standard uncertainties. So, what are the particular sources of each error? Physical and chemical laboratory experiments include three primary sources of error:. Solubility Lab Sources Of Error.
From studylib.net
Solubility Rules Lab Solubility Lab Sources Of Error So, what are the particular sources of each error? Systematic error, random error and human error. By the end of this lab, students should be able to: Properly use an analytical balance to measure mass. These sources of errors in lab should be studied well before any further action. One example of a systematic error could be using a ph. Solubility Lab Sources Of Error.
From www.chegg.com
Solved Experiment 9 Determination of the SolubilityProduct Solubility Lab Sources Of Error Systematic error, random error and human error. Several factors can cause errors in titration findings, including misreading volumes, mistaken concentration values or faulty technique. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Properly use an analytical balance to measure mass.. Solubility Lab Sources Of Error.
From studylib.net
Solubility Rules Lab Solubility Lab Sources Of Error Simplify by grouping sources, quantify sources and convert components to standard uncertainties. Systematic error, random error and human error. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. The simplest way to deal with errors and inaccuracy in a quantitative manner. Solubility Lab Sources Of Error.
From studylib.net
Solubility Rules Lab Varga Solubility Lab Sources Of Error Simplify by grouping sources, quantify sources and convert components to standard uncertainties. So, what are the particular sources of each error? By the end of this lab, students should be able to: Titration is a sensitive analytical method that lets you determine an unknown concentration of a chemical in solution by introducing a known concentration of another chemical. Several factors. Solubility Lab Sources Of Error.
From studylib.net
Solubility Lab Solubility Lab Sources Of Error Systematic error, random error and human error. By the end of this lab, students should be able to: Properly use an analytical balance to measure mass. One example of a systematic error could be using a ph meter that is incorrectly calibrated, so that it reads 6.10 when immersed in a ph 6.00 buffer. Titration is a sensitive analytical method. Solubility Lab Sources Of Error.
From iu.pressbooks.pub
Effect of Temperature and Solvent on Solubility IU East Experimental Solubility Lab Sources Of Error Set up an experimental work station to. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. These sources. Solubility Lab Sources Of Error.
From www.chegg.com
REPORT SHEET Determination of the SolubilityProduct Solubility Lab Sources Of Error These sources of errors in lab should be studied well before any further action. Simplify by grouping sources, quantify sources and convert components to standard uncertainties. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. Set up an experimental work station to. One example of a. Solubility Lab Sources Of Error.
From oneclass.com
OneClass REPORT SHEET EXPERIMENT Determination of the 27 Solubility Solubility Lab Sources Of Error Physical and chemical laboratory experiments include three primary sources of error: Set up an experimental work station to. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. One example of a systematic error could be using a ph meter that is. Solubility Lab Sources Of Error.
From www.studypool.com
SOLUTION Determination of Solubility Product Constant of Calcium Solubility Lab Sources Of Error Simplify by grouping sources, quantify sources and convert components to standard uncertainties. The simplest way to deal with errors and inaccuracy in a quantitative manner is to convert all of the estimated errors into percentage. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement. Solubility Lab Sources Of Error.