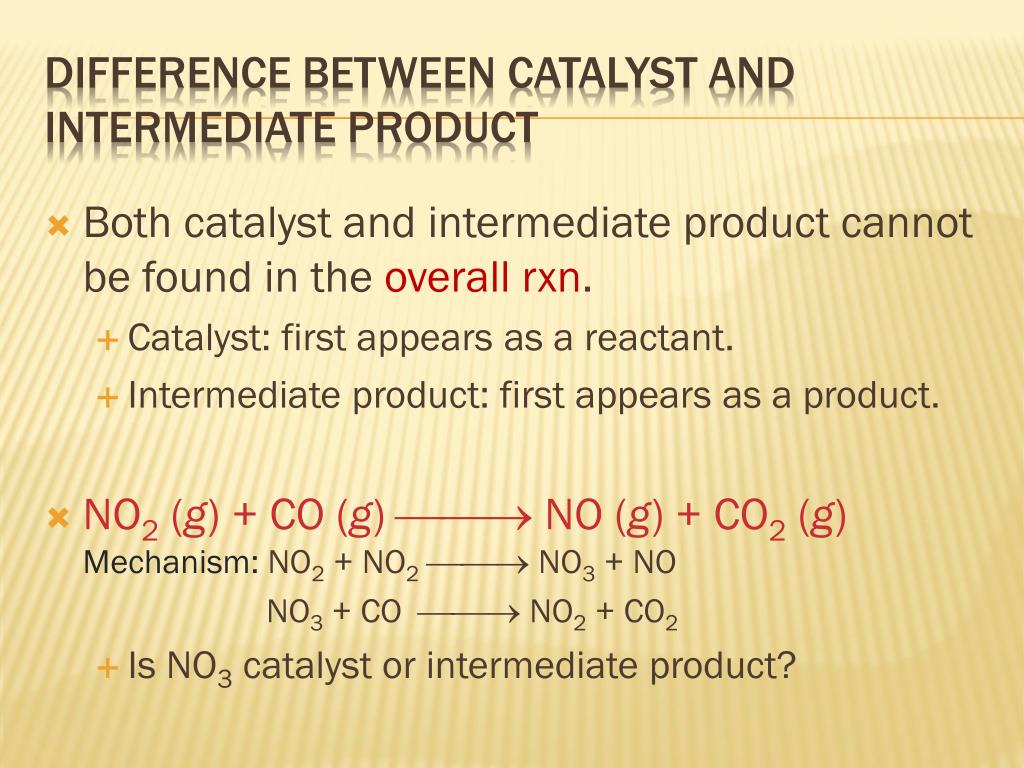
Catalyst And Intermediate Difference . A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is used at the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. Goes over two examples that highlight the differences between catalysts and intermediates. It provides an alternative pathway for the reaction by reducing the. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable.
from www.slideserve.com
It provides an alternative pathway for the reaction by reducing the. A catalyst is used at the. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. Goes over two examples that highlight the differences between catalysts and intermediates. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work.
PPT Unit 46 Rxn mechanisms PowerPoint Presentation, free download
Catalyst And Intermediate Difference To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is used at the. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. Goes over two examples that highlight the differences between catalysts and intermediates. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. It provides an alternative pathway for the reaction by reducing the.
From www.organicchemistrytutor.com
What is the Difference Between a Transition State and an Intermediate Catalyst And Intermediate Difference To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. Goes over two examples that highlight the differences between catalysts and intermediates. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. This page looks at the the different types of catalyst. Catalyst And Intermediate Difference.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalyst And Intermediate Difference It provides an alternative pathway for the reaction by reducing the. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. To. Catalyst And Intermediate Difference.
From chemistnotes.com
Reaction Intermediates, Example, and Types Chemistry Notes Catalyst And Intermediate Difference It provides an alternative pathway for the reaction by reducing the. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. Goes over two examples that highlight the differences between catalysts and. Catalyst And Intermediate Difference.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalyst And Intermediate Difference Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. Goes over two examples that highlight. Catalyst And Intermediate Difference.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalyst And Intermediate Difference To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. It provides an alternative pathway for the reaction by reducing the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalysts are reusable. Catalyst And Intermediate Difference.
From lavelle.chem.ucla.edu
Intermediate vs. Catalyst CHEMISTRY COMMUNITY Catalyst And Intermediate Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. A catalyst is used. Catalyst And Intermediate Difference.
From www.slideserve.com
PPT Homogeneous Catalysis Introduction PowerPoint Presentation Catalyst And Intermediate Difference Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This chemistry video tutorial. Catalyst And Intermediate Difference.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free Catalyst And Intermediate Difference A catalyst is a chemical compound that increases the rate of a reaction without being consumed. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a. Catalyst And Intermediate Difference.
From slideplayer.com
Equilibrium Chapter ppt download Catalyst And Intermediate Difference A catalyst is used at the. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind,. Catalyst And Intermediate Difference.
From tagvault.org
Catalyst vs Intermediate (Explained) Catalyst And Intermediate Difference Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is used at the. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that increases the rate of a chemical reaction without. Catalyst And Intermediate Difference.
From inspiritvr.com
CBSE Class 12 Chemistry Chapter 5 Revision Notes Inspirit Catalyst And Intermediate Difference A catalyst is a chemical compound that increases the rate of a reaction without being consumed. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. A catalyst is used at the.. Catalyst And Intermediate Difference.
From www.youtube.com
Practice Identifying Catalysts and Intermediates YouTube Catalyst And Intermediate Difference A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. Goes over. Catalyst And Intermediate Difference.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalyst And Intermediate Difference It provides an alternative pathway for the reaction by reducing the. A catalyst is used at the. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. Goes over two examples that highlight the differences between catalysts and intermediates. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence. Catalyst And Intermediate Difference.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst Catalyst And Intermediate Difference This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. Catalysts are reusable and can selectively promote specific. Catalyst And Intermediate Difference.
From www.differencebetween.com
Difference Between Catalytic and Stoichiometric Reagents Compare the Catalyst And Intermediate Difference Goes over two examples that highlight the differences between catalysts and intermediates. It provides an alternative pathway for the reaction by reducing the. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of. Catalyst And Intermediate Difference.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism Catalyst And Intermediate Difference This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. A catalyst is a substance that increases the rate of a chemical. Catalyst And Intermediate Difference.
From www.slideserve.com
PPT Part V Reaction Mechanisms PowerPoint Presentation Catalyst And Intermediate Difference Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a. Catalyst And Intermediate Difference.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalyst And Intermediate Difference A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. Goes over. Catalyst And Intermediate Difference.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalyst And Intermediate Difference It provides an alternative pathway for the reaction by reducing the. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. A catalyst is a chemical compound that increases the rate of a reaction. Catalyst And Intermediate Difference.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Catalyst And Intermediate Difference A catalyst is a chemical compound that increases the rate of a reaction without being consumed. A catalyst is used at the. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate. Catalyst And Intermediate Difference.
From www.studyorgo.com
Energy Diagram Module Series Part Three Intermediates and Rate Catalyst And Intermediate Difference A catalyst is a chemical compound that increases the rate of a reaction without being consumed. Goes over two examples that highlight the differences between catalysts and intermediates. A catalyst is used at the. It provides an alternative pathway for the reaction by reducing the. To summarize, a catalyst is a substance that speeds up a reaction without being consumed,. Catalyst And Intermediate Difference.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Catalyst And Intermediate Difference Goes over two examples that highlight the differences between catalysts and intermediates. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. It provides. Catalyst And Intermediate Difference.
From www.pinterest.com
Catalyst vs Intermediate Tabular Form Functional group, Side Catalyst And Intermediate Difference A catalyst is used at the. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate. Catalyst And Intermediate Difference.
From courses.lumenlearning.com
Catalysis Chemistry for Majors Catalyst And Intermediate Difference Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is used at the. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind,. Catalyst And Intermediate Difference.
From 2012books.lardbucket.org
Catalysis Catalyst And Intermediate Difference Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is used at the. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. This page looks at the the. Catalyst And Intermediate Difference.
From slideplayer.com
Reaction Mechanism The reaction mechanism is the series of elementary Catalyst And Intermediate Difference A catalyst is a chemical compound that increases the rate of a reaction without being consumed. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. A catalyst is a substance that. Catalyst And Intermediate Difference.
From www.slideserve.com
PPT Unit 46 Rxn mechanisms PowerPoint Presentation, free download Catalyst And Intermediate Difference A catalyst is used at the. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind,. Catalyst And Intermediate Difference.
From www.youtube.com
Identifying Catalyst and Intermediates in Elementary Steps of Catalyst And Intermediate Difference This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is used. Catalyst And Intermediate Difference.
From www.youtube.com
ChemDoctor Reaction Mechanisms 3 Homogeneous catalysts and Catalyst And Intermediate Difference Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is used at the. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. A catalyst is a substance that increases the rate of a chemical reaction without itself being. Catalyst And Intermediate Difference.
From www.ck12.org
Reaction Mechanisms Example 3 ( Video ) Chemistry CK12 Foundation Catalyst And Intermediate Difference It provides an alternative pathway for the reaction by reducing the. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. Goes over two examples that highlight the differences between catalysts and intermediates. A catalyst is used at the. To summarize, a catalyst is a substance that speeds up a reaction without being consumed,. Catalyst And Intermediate Difference.
From www.finechemical.net
Catalyst vs Intermediate What is the difference? Catalyst And Intermediate Difference A catalyst is used at the. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. It provides an alternative pathway for the reaction by reducing the. Goes over two examples that highlight the. Catalyst And Intermediate Difference.
From www.researchgate.net
Classification and summary of different catalysts. Download Catalyst And Intermediate Difference It provides an alternative pathway for the reaction by reducing the. Goes over two examples that highlight the differences between catalysts and intermediates. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of. Catalyst And Intermediate Difference.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalyst And Intermediate Difference A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. Catalysts are reusable and can selectively promote specific reactions, while intermediates are consumed and influence reaction. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. This chemistry video tutorial explains how to identify. Catalyst And Intermediate Difference.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation Catalyst And Intermediate Difference A catalyst is a substance that increases the rate of a chemical reaction without itself being changed in the process. Goes over two examples that highlight the differences between catalysts and intermediates. To summarize, a catalyst is a substance that speeds up a reaction without being consumed, while an intermediate is a temporary, unstable. A catalyst is a chemical compound. Catalyst And Intermediate Difference.
From www.youtube.com
R2.2.6 Intermediates and catalysts (HL) YouTube Catalyst And Intermediate Difference It provides an alternative pathway for the reaction by reducing the. This chemistry video tutorial explains how to identify the intermediate and the catalyst in a reaction. Goes over two examples that highlight the differences between catalysts and intermediates. A catalyst is a chemical compound that increases the rate of a reaction without being consumed. To summarize, a catalyst is. Catalyst And Intermediate Difference.