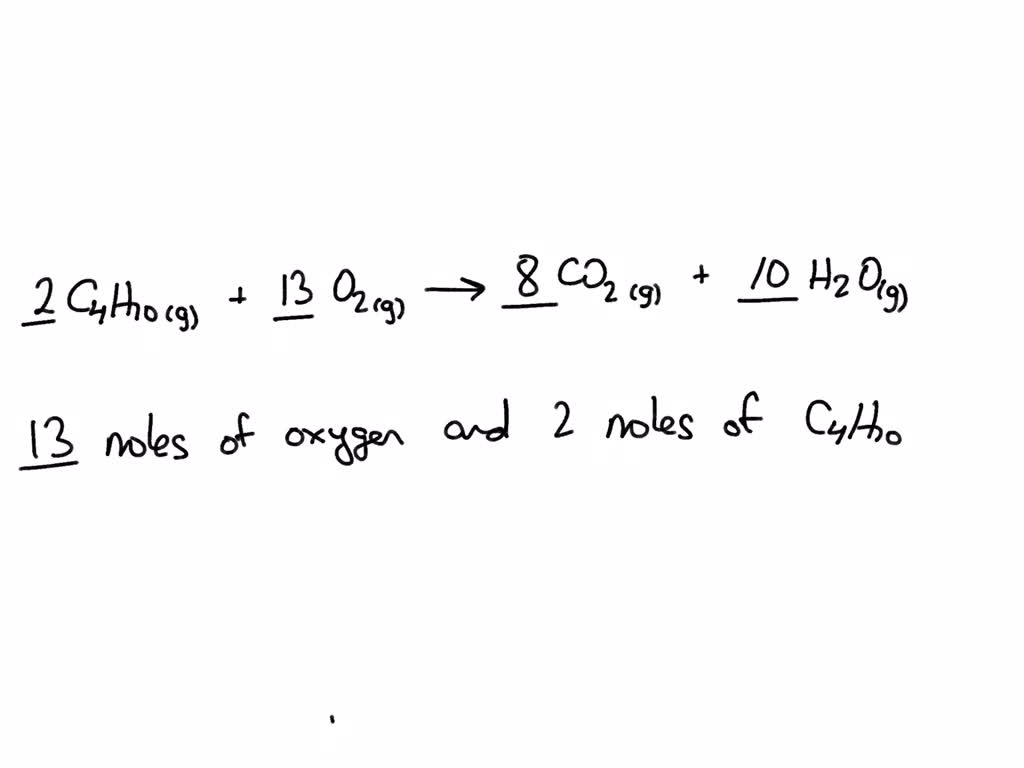Butane Reaction With Oxygen . Butane releases its chemical energy by undergoing hydrocarbon combustion. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Below is a hydrocarbon combustion. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. butane + oxygen → carbon dioxide + water. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water.
from www.numerade.com
butane + oxygen → carbon dioxide + water. Butane releases its chemical energy by undergoing hydrocarbon combustion. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Below is a hydrocarbon combustion.
SOLVED The balanced chemical equation for the reaction between butane
Butane Reaction With Oxygen this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. Butane releases its chemical energy by undergoing hydrocarbon combustion. Below is a hydrocarbon combustion. butane + oxygen → carbon dioxide + water. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two.
From www.numerade.com
SOLVED When butane (C4H10) reacts with oxygen, carbon dioxide and Butane Reaction With Oxygen With butane (\(\ce{c4h10}\)), you can again balance the carbons and. butane + oxygen → carbon dioxide + water. Butane releases its chemical energy by undergoing hydrocarbon combustion. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. if. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED Write a balanced chemical reaction from each of the following Butane Reaction With Oxygen With butane (\(\ce{c4h10}\)), you can again balance the carbons and. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. Below is a hydrocarbon combustion. in order to balance c4h10 + o2. Butane Reaction With Oxygen.
From www.pinterest.com
Black = carbon Red = oxygen White = hydrogen Presented is the reaction Butane Reaction With Oxygen Below is a hydrocarbon combustion. Butane releases its chemical energy by undergoing hydrocarbon combustion. butane + oxygen → carbon dioxide + water. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. With butane (\(\ce{c4h10}\)), you can again balance. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED Butane is the liquid found in lighter fluid. If it reacts with Butane Reaction With Oxygen this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. Below is a hydrocarbon combustion. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. C4h 10(g) + 13 2 o2(g) → 4co2(g). Butane Reaction With Oxygen.
From wizedu.com
Gaseous butane CH3CH22CH3 reacts with gaseous oxygen gas O2 to produce Butane Reaction With Oxygen Below is a hydrocarbon combustion. butane + oxygen → carbon dioxide + water. Butane releases its chemical energy by undergoing hydrocarbon combustion. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen.. Butane Reaction With Oxygen.
From www.coursehero.com
[Solved] Gaseous butane CH3CH22CH3 reacts with gaseous oxygen gas O2 to Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. Below is a hydrocarbon combustion. butane + oxygen → carbon dioxide. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between butane Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. butane + oxygen → carbon dioxide + water. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. in. Butane Reaction With Oxygen.
From www.chegg.com
Solved Butane C4H 10. reacts with oxygen. O2. to form water. Butane Reaction With Oxygen this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. butane + oxygen → carbon dioxide + water. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. Butane releases its chemical energy by undergoing hydrocarbon combustion. C4h 10(g) + 13 2. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED Gaseous butane will react with gaseous oxygen to produce Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. butane + oxygen → carbon dioxide + water. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. in order to balance c4h10 + o2 = co2 + h2o you'll need. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED When butane (C4H10) reacts with oxygen, carbon dioxide Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. Butane releases its chemical energy by undergoing hydrocarbon combustion. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. C4h 10(g) +. Butane Reaction With Oxygen.
From oneclass.com
OneClass Gaseous butane, CH_2(CH_2)_2CH_3 reacts with gaseous oxygen Butane Reaction With Oxygen butane + oxygen → carbon dioxide + water. Below is a hydrocarbon combustion. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. Butane releases its chemical energy by undergoing hydrocarbon combustion.. Butane Reaction With Oxygen.
From treatybottle13.pythonanywhere.com
Marvelous Balanced Equation For Butane And Oxygen Formula Photosynthesis Butane Reaction With Oxygen butane + oxygen → carbon dioxide + water. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. Below is a hydrocarbon combustion. Butane releases its chemical energy by undergoing hydrocarbon combustion. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. C4h 10(g) + 13 2 o2(g) → 4co2(g). Butane Reaction With Oxygen.
From www.chegg.com
Solved Gaseous butane, CH_3(CH_2)_2CH_3 reacts with gaseous Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Butane releases its chemical energy by undergoing hydrocarbon combustion. Below is a hydrocarbon combustion. butane + oxygen → carbon dioxide + water. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. this unbalanced equation represents the. Butane Reaction With Oxygen.
From byjus.com
If isobutane and n butane are present in a gas , then how much oxygen Butane Reaction With Oxygen With butane (\(\ce{c4h10}\)), you can again balance the carbons and. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Butane releases its chemical energy by undergoing hydrocarbon combustion. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. butane + oxygen → carbon dioxide + water. Below. Butane Reaction With Oxygen.
From www.numerade.com
When butane burns, it combines with oxygen in the… Butane Reaction With Oxygen butane + oxygen → carbon dioxide + water. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Below is a hydrocarbon combustion. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED The combustion of butane gas, C4H10 in oxygen gas, O2, produces Butane Reaction With Oxygen Butane releases its chemical energy by undergoing hydrocarbon combustion. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. With butane. Butane Reaction With Oxygen.
From solvedlib.com
Gaseous butane CH3CH22CH3 reacts with gaseous oxygen … SolvedLib Butane Reaction With Oxygen With butane (\(\ce{c4h10}\)), you can again balance the carbons and. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. butane + oxygen → carbon dioxide + water. Butane releases its chemical energy by undergoing hydrocarbon combustion. if the liquid is not very volatile, only those molecules on the surface. Butane Reaction With Oxygen.
From www.coursehero.com
[Solved] Gaseous butane ( CH3(CH2), CH3 ) reacts with gaseous oxygen Butane Reaction With Oxygen this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. Butane releases its chemical energy by undergoing hydrocarbon combustion. butane + oxygen → carbon dioxide + water. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. in order to balance c4h10 + o2 = co2 + h2o. Butane Reaction With Oxygen.
From questions.kunduz.com
Gaseous butane (CH; (CH2),CH3) will react Organic Chemistry Butane Reaction With Oxygen C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. butane + oxygen → carbon dioxide + water. Below. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED Gaseous butane (CH3CH22CH3) reacts with gaseous oxygen gas (O2 Butane Reaction With Oxygen With butane (\(\ce{c4h10}\)), you can again balance the carbons and. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. Butane releases its chemical energy by undergoing hydrocarbon combustion. butane + oxygen → carbon dioxide + water. if the liquid is not very volatile, only those molecules on the surface. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED Gaseous butane (CH3(CH2)2CH3) reacts with gaseous oxygen gas Butane Reaction With Oxygen butane + oxygen → carbon dioxide + water. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. Butane releases. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED According to the following reaction, how many moles of carbon Butane Reaction With Oxygen With butane (\(\ce{c4h10}\)), you can again balance the carbons and. Butane releases its chemical energy by undergoing hydrocarbon combustion. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Below is a hydrocarbon. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED When butane (C4H10) reacts with oxygen, carbon dioxide and Butane Reaction With Oxygen Below is a hydrocarbon combustion. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. butane + oxygen → carbon dioxide + water. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. Butane releases its chemical energy by undergoing hydrocarbon combustion. if the liquid is not very volatile, only those molecules on the surface can. Butane Reaction With Oxygen.
From www.chegg.com
Solved Gaseous butane (CH3(CH2)2CH3) reacts with gaseous Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. Butane releases its chemical energy by undergoing hydrocarbon combustion. in. Butane Reaction With Oxygen.
From solvedlib.com
Gaseous butane (CH3CH3) reacts with gaseous oxygen ga… SolvedLib Butane Reaction With Oxygen Below is a hydrocarbon combustion. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. this unbalanced equation represents the complete. Butane Reaction With Oxygen.
From www.youtube.com
Balance combustion Butane 20pct excess 85pct consumed YouTube Butane Reaction With Oxygen With butane (\(\ce{c4h10}\)), you can again balance the carbons and. butane + oxygen → carbon dioxide + water. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. Butane releases its chemical energy by undergoing hydrocarbon combustion. if the liquid is not very volatile, only those molecules on the. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED write a balanced equation showing the reaction of butane with Butane Reaction With Oxygen C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. Below is a hydrocarbon combustion. butane + oxygen → carbon. Butane Reaction With Oxygen.
From www.wou.edu
Chapter 6 Quantities in Chemical Reactions Chemistry Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Butane releases its chemical energy by undergoing hydrocarbon combustion. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. Below is a. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED Gaseous butane CH,( CHz CH; will react with gaseous oxygen Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. Butane releases its chemical energy by undergoing hydrocarbon combustion. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. in. Butane Reaction With Oxygen.
From solvedlib.com
Gaseous butane CH3(CH2)2CH3 reacts with gaseous oxyge… SolvedLib Butane Reaction With Oxygen butane + oxygen → carbon dioxide + water. Butane releases its chemical energy by undergoing hydrocarbon combustion. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. C4h 10(g) + 13 2. Butane Reaction With Oxygen.
From www.researchgate.net
Reaction pathway for the selective oxidation of butane over the OMo Butane Reaction With Oxygen in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. Butane releases its chemical energy by undergoing hydrocarbon combustion. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. butane +. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED Gaseous butane CH3CH22CH3 will react with gaseous oxygen O2 to Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. Below is a hydrocarbon combustion. Butane releases its chemical energy by undergoing hydrocarbon combustion. With butane (\(\ce{c4h10}\)), you can again balance the carbons. Butane Reaction With Oxygen.
From www.chegg.com
Solved Butane (C_4 H_10) Reacts With Oxygen Gas To Produc... Butane Reaction With Oxygen butane + oxygen → carbon dioxide + water. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. this unbalanced equation represents the complete combustion of butane in oxygen to produce carbon dioxide and water. if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. With butane. Butane Reaction With Oxygen.
From www.numerade.com
SOLVED Butane (C4H10) reacts with oxygen to form carbon dioxide and Butane Reaction With Oxygen Below is a hydrocarbon combustion. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two. Butane releases its chemical energy by undergoing hydrocarbon combustion. butane + oxygen → carbon dioxide + water. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. if the liquid is not very volatile,. Butane Reaction With Oxygen.
From www.doubtnut.com
Butane (C(4)H(10)) gas burns in oxygen to give carbon dioxide and water Butane Reaction With Oxygen if the liquid is not very volatile, only those molecules on the surface can react with the oxygen. With butane (\(\ce{c4h10}\)), you can again balance the carbons and. C4h 10(g) + 13 2 o2(g) → 4co2(g) +5h 2o(g) is the. in order to balance c4h10 + o2 = co2 + h2o you'll need to watch out for two.. Butane Reaction With Oxygen.