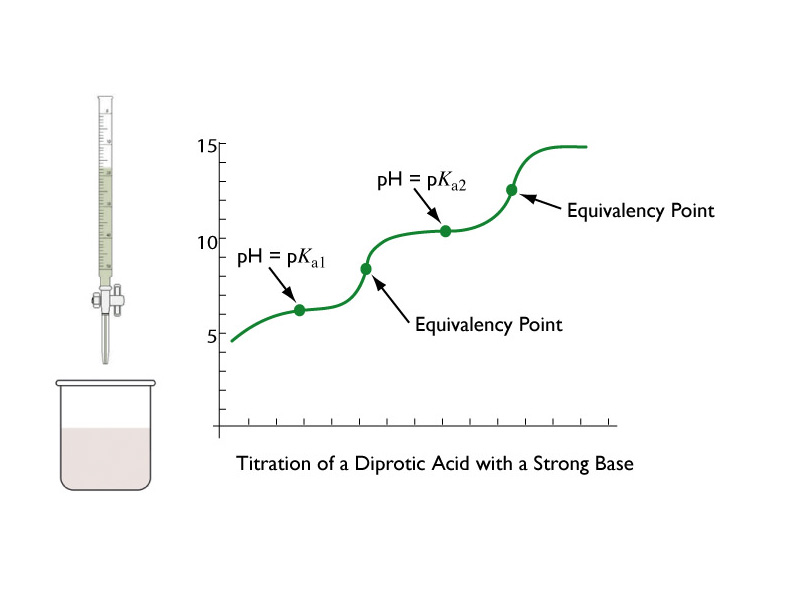
Titration Curve Diprotic Acid . The common example of this would be ethanoic acid and ammonia. Titration curves for weak acid v weak base. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Determine acid ka (base kb) by chapter 11 section: Each titration curve is for 50.0 ml of 0.0500 m. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. Ph = pka + log ([base]/[acid] Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. Titration curve of a weak diprotic acid. Titration is used to calculate acid dissociation constants. This figure shows the basic features of a titration curve of a weak polyprotic acid. It so happens that these two are. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible.
from mavink.com
Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. The common example of this would be ethanoic acid and ammonia. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. Titration curves for weak acid v weak base. Each titration curve is for 50.0 ml of 0.0500 m. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Titration is used to calculate acid dissociation constants. Titration curve of a weak diprotic acid. Ph = pka + log ([base]/[acid]
Diprotic Acid Titration Curve
Titration Curve Diprotic Acid It so happens that these two are. Ph = pka + log ([base]/[acid] Titration curves for weak acid v weak base. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. Titration curve of a weak diprotic acid. Determine acid ka (base kb) by chapter 11 section: Titration is used to calculate acid dissociation constants. This figure shows the basic features of a titration curve of a weak polyprotic acid. Each titration curve is for 50.0 ml of 0.0500 m. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. It so happens that these two are. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. The common example of this would be ethanoic acid and ammonia. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Curve Diprotic Acid Determine acid ka (base kb) by chapter 11 section: It so happens that these two are. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Titration curves for weak acid v weak base. Ph = pka + log ([base]/[acid]. Titration Curve Diprotic Acid.
From chemwiki.ucdavis.edu
9B AcidBase Titrations Chemwiki Titration Curve Diprotic Acid Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. Determine acid ka (base kb) by chapter 11 section: Titration is used to calculate acid dissociation constants. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid,. Titration Curve Diprotic Acid.
From mavink.com
Diprotic Acid Titration Curve Titration Curve Diprotic Acid Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. This figure shows the basic features of a titration curve of a weak polyprotic acid. It so happens that these two are. Determine. Titration Curve Diprotic Acid.
From www.youtube.com
Titration calculations and diprotic acid curves YouTube Titration Curve Diprotic Acid Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. This figure shows the basic features of a titration curve of a weak polyprotic acid. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Titration curve of a weak diprotic acid. The common example of this would. Titration Curve Diprotic Acid.
From www.chegg.com
longrightarrow weak base1a. What is the pH of a Titration Curve Diprotic Acid Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. This figure shows the basic features of a titration curve of a weak polyprotic acid. It so happens that these two are. Titration curve of a weak diprotic acid. Titration. Titration Curve Diprotic Acid.
From mavink.com
Diprotic Acid Titration Curve Titration Curve Diprotic Acid Titration curves for weak acid v weak base. Determine acid ka (base kb) by chapter 11 section: This figure shows the basic features of a titration curve of a weak polyprotic acid. Titration is used to calculate acid dissociation constants. The common example of this would be ethanoic acid and ammonia. In this jc2 webinar we want to learn how. Titration Curve Diprotic Acid.
From socratic.org
What should I know about a titration curve of strong base added into Titration Curve Diprotic Acid Titration is used to calculate acid dissociation constants. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. The common example of this would be ethanoic acid and ammonia. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Figure 9.11 titration curves for the diprotic weak acids maleic acid,. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid The common example of this would be ethanoic acid and ammonia. Ph = pka + log ([base]/[acid] Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. It so happens that these two are. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. Each titration curve is for 50.0. Titration Curve Diprotic Acid.
From www.pearson.com
McMurry Chemistry 8th Edition Chapter 17 Problem 43 Titration Curve Diprotic Acid Ph = pka + log ([base]/[acid] This figure shows the basic features of a titration curve of a weak polyprotic acid. It so happens that these two are. Titration curve of a weak diprotic acid. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. The common example of this would be ethanoic acid. Titration Curve Diprotic Acid.
From philschatz.com
AcidBase Titrations · Chemistry Titration Curve Diprotic Acid Titration is used to calculate acid dissociation constants. Ph = pka + log ([base]/[acid] This figure shows the basic features of a titration curve of a weak polyprotic acid. Determine acid ka (base kb) by chapter 11 section: Titration curve of a weak diprotic acid. Titration curves for weak acid v weak base. Each titration curve is for 50.0 ml. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. It so happens that these two are. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. The common. Titration Curve Diprotic Acid.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Diprotic Acid Titration curve of a weak diprotic acid. Ph = pka + log ([base]/[acid] Titration is used to calculate acid dissociation constants. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. This figure shows the basic features of a titration curve of a weak polyprotic acid. Titration curves for weak acid v weak base. Figure 9.11. Titration Curve Diprotic Acid.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Diprotic Acid A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. Determine acid ka (base kb) by chapter 11 section: It so happens that these two are. Titration is used to calculate acid dissociation constants. In this jc2 webinar we want to learn how to sketch titration curve for diprotic. Titration Curve Diprotic Acid.
From socratic.org
Titration curve? Please help... Socratic Titration Curve Diprotic Acid A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. Ph = pka + log ([base]/[acid] This figure shows the basic features of a titration curve of a weak polyprotic acid. Each titration curve is for 50.0 ml of 0.0500 m. Figure 9.11 titration curves for the diprotic weak. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. It so happens that these two are. Ph = pka + log ([base]/[acid] This figure shows the basic features of a titration curve of a. Titration Curve Diprotic Acid.
From mungfali.com
Buffer Region On Titration Curve Titration Curve Diprotic Acid Titration curve of a weak diprotic acid. It so happens that these two are. Each titration curve is for 50.0 ml of 0.0500 m. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. In this jc2 webinar we want to learn how to sketch titration curve for diprotic. Titration Curve Diprotic Acid.
From dokumen.tips
(PDF) Calculation of neutralization enthalpies from thermometric Titration Curve Diprotic Acid This figure shows the basic features of a titration curve of a weak polyprotic acid. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. It so happens that these two are. Titration curves for weak acid v weak base. Determine acid ka (base kb) by chapter 11 section:. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid It so happens that these two are. Ph = pka + log ([base]/[acid] Titration is used to calculate acid dissociation constants. Each titration curve is for 50.0 ml of 0.0500 m. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Titration curve of a weak diprotic acid. A typical titration curve of a. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid Titration curves for weak acid v weak base. Each titration curve is for 50.0 ml of 0.0500 m. This figure shows the basic features of a titration curve of a weak polyprotic acid. Titration is used to calculate acid dissociation constants. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Figure 9.11 titration. Titration Curve Diprotic Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Curve Diprotic Acid Titration curve of a weak diprotic acid. Determine acid ka (base kb) by chapter 11 section: Ph = pka + log ([base]/[acid] The common example of this would be ethanoic acid and ammonia. It so happens that these two are. Titration is used to calculate acid dissociation constants. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic. Titration Curve Diprotic Acid.
From www.differencebetween.com
What is the Difference Between Monoprotic and Diprotic Acid Compare Titration Curve Diprotic Acid Titration is used to calculate acid dissociation constants. The common example of this would be ethanoic acid and ammonia. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. Determine acid ka (base kb) by chapter 11 section:. Titration Curve Diprotic Acid.
From forums.studentdoctor.net
Confusion about titration of polyprotic acids Student Doctor Network Titration Curve Diprotic Acid Each titration curve is for 50.0 ml of 0.0500 m. This figure shows the basic features of a titration curve of a weak polyprotic acid. It so happens that these two are. Titration is used to calculate acid dissociation constants. Ph = pka + log ([base]/[acid] Titration curves for weak acid v weak base. Titration curve of a weak diprotic. Titration Curve Diprotic Acid.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Diprotic Acid The common example of this would be ethanoic acid and ammonia. This figure shows the basic features of a titration curve of a weak polyprotic acid. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. Titration is used to calculate acid dissociation constants. Figure 9.11 titration curves for. Titration Curve Diprotic Acid.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记5.2.6 Titration Curves翰林国际教育 Titration Curve Diprotic Acid The common example of this would be ethanoic acid and ammonia. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Titration is used. Titration Curve Diprotic Acid.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Curve Diprotic Acid Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Titration is used to calculate acid dissociation constants. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. Titration curve of a weak diprotic. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid The common example of this would be ethanoic acid and ammonia. Titration curves for weak acid v weak base. It so happens that these two are. In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. Each titration curve is. Titration Curve Diprotic Acid.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Diprotic Acid A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. Titration curves for weak acid v weak base. Each titration curve is for 50.0 ml of 0.0500 m. Determine acid ka (base kb) by chapter 11 section: Titration is used to calculate acid dissociation constants. In this jc2 webinar. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid This figure shows the basic features of a titration curve of a weak polyprotic acid. The common example of this would be ethanoic acid and ammonia. Determine acid ka (base kb) by chapter 11 section: Each titration curve is for 50.0 ml of 0.0500 m. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid Titration is used to calculate acid dissociation constants. The common example of this would be ethanoic acid and ammonia. Titration curves for weak acid v weak base. It so happens that these two are. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. Titration curve of a weak diprotic acid. Ph = pka + log. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid In this jc2 webinar we want to learn how to sketch titration curve for diprotic acid. Each titration curve is for 50.0 ml of 0.0500 m. Determine acid ka (base kb) by chapter 11 section: It so happens that these two are. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. A typical. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid Titration curves for weak acid v weak base. Titration curve of a weak diprotic acid. Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. It so happens that these two are. Ph = pka + log ([base]/[acid] The common example of this would be ethanoic acid and ammonia. Determine acid ka (base kb). Titration Curve Diprotic Acid.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curve Diprotic Acid Titration is used to calculate acid dissociation constants. Let's consider the titration of ethanedioic acid (h 2 c 2 o 4) with. The common example of this would be ethanoic acid and ammonia. Titration curve of a weak diprotic acid. This figure shows the basic features of a titration curve of a weak polyprotic acid. Figure 9.11 titration curves for. Titration Curve Diprotic Acid.
From ar.inspiredpencil.com
Titration Curve Diprotic Acid Titration Curve Diprotic Acid Figure 9.11 titration curves for the diprotic weak acids maleic acid, malonic acid, and succinic acid. The common example of this would be ethanoic acid and ammonia. Ph = pka + log ([base]/[acid] Titration curve of a weak diprotic acid. It so happens that these two are. Each titration curve is for 50.0 ml of 0.0500 m. Determine acid ka. Titration Curve Diprotic Acid.
From quizlet.com
Diprotic Acid, H2A Titration Curve Diagram Quizlet Titration Curve Diprotic Acid Titration curve of a weak diprotic acid. The common example of this would be ethanoic acid and ammonia. Each titration curve is for 50.0 ml of 0.0500 m. Titration curves for weak acid v weak base. This figure shows the basic features of a titration curve of a weak polyprotic acid. A typical titration curve of a diprotic acid, oxalic. Titration Curve Diprotic Acid.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Curve Diprotic Acid Determine acid ka (base kb) by chapter 11 section: Titration curves for weak acid v weak base. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide.both equivalence points are visible. It so happens that these two are. This figure shows the basic features of a titration curve of a weak polyprotic acid.. Titration Curve Diprotic Acid.