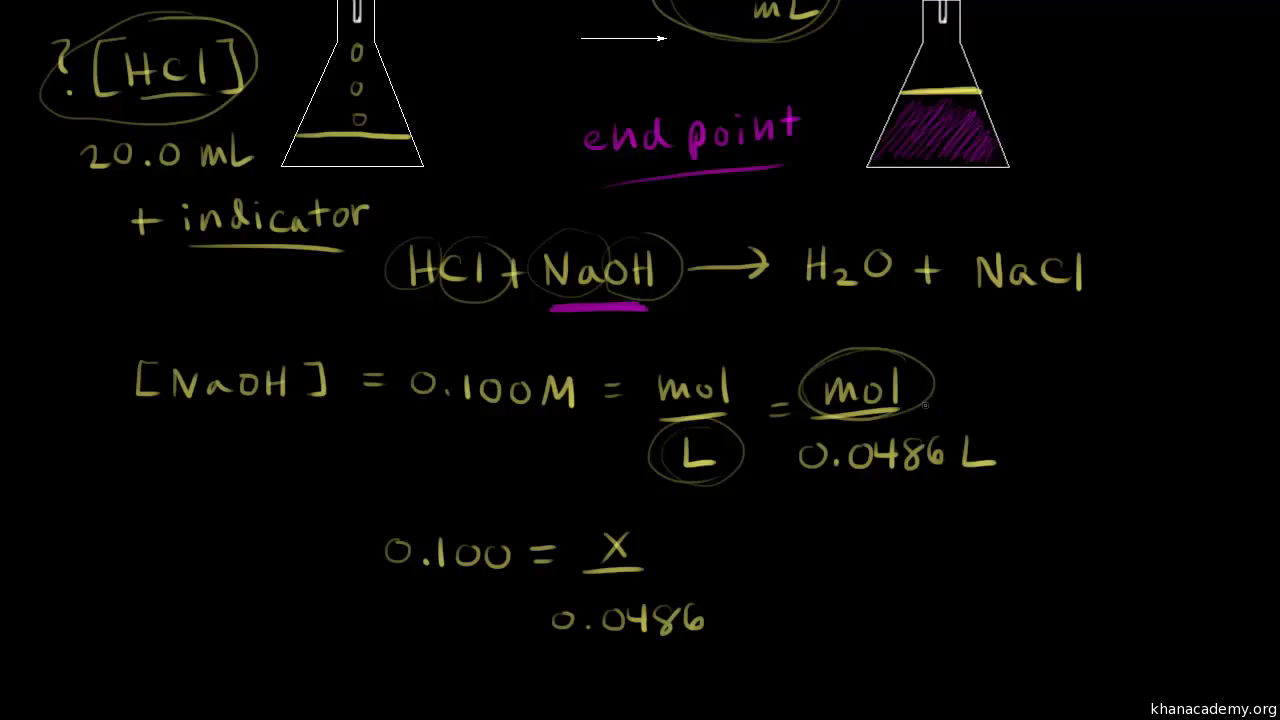End Point Simple Definition Chemistry . Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Titrations with a ph probe. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Finding the endpoint of a titration. Using a ph probe and using a colour indicator. The end point is where the titration ends in practice. The end point typically comes straight after the equivalence point, which is the ideal point The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change.
from ar.inspiredpencil.com
The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Finding the endpoint of a titration. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. Titrations with a ph probe. The end point typically comes straight after the equivalence point, which is the ideal point The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Using a ph probe and using a colour indicator.
Endpoint Chemistry
End Point Simple Definition Chemistry Using a ph probe and using a colour indicator. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The end point typically comes straight after the equivalence point, which is the ideal point The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titrations with a ph probe. Finding the endpoint of a titration. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Using a ph probe and using a colour indicator. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The end point is where the titration ends in practice.
From gioluaslm.blob.core.windows.net
Security Endpoint Definition at Cynthia Drummond blog End Point Simple Definition Chemistry Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The closer the end point to the equivalence point the better, but it is. End Point Simple Definition Chemistry.
From www.researchgate.net
The trends for endpoint pH values of the leaching solutions under the End Point Simple Definition Chemistry The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Using a ph probe and using a colour indicator. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The end point. End Point Simple Definition Chemistry.
From sciencenotes.org
What Is a Mixture in Chemistry? Definition and Examples End Point Simple Definition Chemistry Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Finding the endpoint of a titration. The end point typically comes straight after the equivalence point, which is the ideal point The completion of a titration is the end point, detected by some. End Point Simple Definition Chemistry.
From www.pinterest.com
Equivalence Point definition The point in a chemical reaction at which End Point Simple Definition Chemistry The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The end point typically comes straight after the equivalence point, which is the ideal point The end point is where the titration ends in practice. The closer the end point to the equivalence point the better,. End Point Simple Definition Chemistry.
From www.caleva.com
The Importance of End Point Detection Consistency End Point Simple Definition Chemistry The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Using a ph probe and using a colour indicator. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The point. End Point Simple Definition Chemistry.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev End Point Simple Definition Chemistry The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Titrations with a ph probe. The end point is where the titration ends in practice.. End Point Simple Definition Chemistry.
From sciencenotes.org
Saturated Solution Definition in Chemistry End Point Simple Definition Chemistry The end point is where the titration ends in practice. Finding the endpoint of a titration. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs. End Point Simple Definition Chemistry.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID End Point Simple Definition Chemistry Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. The end point typically comes straight after the equivalence point, which is. End Point Simple Definition Chemistry.
From exookkqbv.blob.core.windows.net
Discussion Definition Chemistry at Patricia Nobles blog End Point Simple Definition Chemistry The end point typically comes straight after the equivalence point, which is the ideal point The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. Using a ph probe and using a colour indicator. The completion of a titration is the end point, detected by some type. End Point Simple Definition Chemistry.
From www.conquerhsc.com
HSC Chemistry Module 6 Inquiry Question 3 End Point Simple Definition Chemistry Finding the endpoint of a titration. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The main difference between an equivalence point and. End Point Simple Definition Chemistry.
From ar.inspiredpencil.com
Endpoint Titration End Point Simple Definition Chemistry The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. Finding the endpoint of a titration. The completion of a titration is the end point,. End Point Simple Definition Chemistry.
From github.com
GitHub sigfoxtechradio/sigfoxeplib Sigfox EndPoint library End Point Simple Definition Chemistry The end point typically comes straight after the equivalence point, which is the ideal point The end point is where the titration ends in practice. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. Finding the endpoint of a titration. Titrations. End Point Simple Definition Chemistry.
From www.numerade.com
SOLVED Explain the difference between end point and equivalence point End Point Simple Definition Chemistry For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titrations with a ph probe. The end point is where the titration ends in. End Point Simple Definition Chemistry.
From edurev.in
End Point, Equivalence Point and Indicators Physical Chemistry PDF End Point Simple Definition Chemistry For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Finding the endpoint of a titration. The end point typically comes straight after the equivalence point, which is the ideal point Titrations with a ph probe. The end point is where the titration ends in practice. Titration involves the gradual addition. End Point Simple Definition Chemistry.
From www.vrogue.co
Solved Identify The Limiting Reactant In The Reaction vrogue.co End Point Simple Definition Chemistry The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. For a ph titration (between an acid and a base), there are two common ways. End Point Simple Definition Chemistry.
From www.researchgate.net
(S, B)type Left to the A 1 bounce there is a singular IR endpoint End Point Simple Definition Chemistry Finding the endpoint of a titration. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The completion of a titration is the end point, detected. End Point Simple Definition Chemistry.
From ar.inspiredpencil.com
Endpoint Chemistry End Point Simple Definition Chemistry The end point is where the titration ends in practice. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. Finding the endpoint of a titration. Titrations with a ph probe. The point during a titration when an indicator shows that the amount of reactant necessary for. End Point Simple Definition Chemistry.
From www.credly.com
Endpoint assessment Retail Team Leader Distinction Credly End Point Simple Definition Chemistry For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The end point is where the titration ends in practice. The main difference between. End Point Simple Definition Chemistry.
From www.researchgate.net
Liver transplant survival to study endpoint. Download Scientific Diagram End Point Simple Definition Chemistry The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. The end point is where the titration ends in practice. For a ph titration (between an. End Point Simple Definition Chemistry.
From www.vrogue.co
Difference Between Endpoint And Equivalence Point vrogue.co End Point Simple Definition Chemistry The end point typically comes straight after the equivalence point, which is the ideal point Using a ph probe and using a colour indicator. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Titration involves the gradual addition of a reagent of known concentration, known. End Point Simple Definition Chemistry.
From in.pinterest.com
End Point Flashcard 10th Grade Science Self help book, Easy science End Point Simple Definition Chemistry The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The end point is where the titration ends in practice. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration involves the gradual addition of a reagent of known. End Point Simple Definition Chemistry.
From www.storyofmathematics.com
End Point Definition & Meaning End Point Simple Definition Chemistry The end point is where the titration ends in practice. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The closer the end. End Point Simple Definition Chemistry.
From sciencenotes.org
Condensation Reaction Definition and Examples End Point Simple Definition Chemistry Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Titrations with a ph probe. Using a ph probe and using a colour indicator. The end point typically comes straight after the equivalence point, which is the ideal point The main difference between. End Point Simple Definition Chemistry.
From www.vrogue.co
What Is An Element In Chemistry Definition And Exampl vrogue.co End Point Simple Definition Chemistry The end point typically comes straight after the equivalence point, which is the ideal point Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The closer the end point to the equivalence point the better, but it is often not easy to. End Point Simple Definition Chemistry.
From app.jove.com
Complexometric Titration Overview Analytical Chemistry JoVe End Point Simple Definition Chemistry The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The end point typically comes straight after the equivalence point, which is the ideal point Finding the endpoint of a titration. Using a ph probe and using a colour indicator. The end point is where the titration ends in. End Point Simple Definition Chemistry.
From www.evoluzioneinformatica.net
Sicurezza dell'Endpoint Evoluzione Informatica End Point Simple Definition Chemistry Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. Using a ph probe and using a colour indicator. The end point typically comes straight after the equivalence point, which is the ideal point The completion of a titration is the end point,. End Point Simple Definition Chemistry.
From dev.classmethod.jp
CodeDeployのVPCエンドポイントが必要なケースを整理してみた DevelopersIO End Point Simple Definition Chemistry Finding the endpoint of a titration. Titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be determined, known as the. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. Using a ph probe. End Point Simple Definition Chemistry.
From ar.inspiredpencil.com
Endpoint Chemistry End Point Simple Definition Chemistry The end point typically comes straight after the equivalence point, which is the ideal point The end point is where the titration ends in practice. The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. The completion of a titration is the end point, detected by some type of. End Point Simple Definition Chemistry.
From www.youtube.com
What is End Point, and Fixed Time Reaction [English End Point Simple Definition Chemistry For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The end point typically comes straight after the equivalence point, which is the ideal point The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Titration involves the gradual addition. End Point Simple Definition Chemistry.
From fyogwmjbh.blob.core.windows.net
Reflux Condenser Chemistry Definition at Lisa Morse blog End Point Simple Definition Chemistry The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. The main difference between an equivalence point and an endpoint is that. End Point Simple Definition Chemistry.
From ar.inspiredpencil.com
Endpoint Chemistry End Point Simple Definition Chemistry Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. Titrations with a ph probe. The completion of a titration is. End Point Simple Definition Chemistry.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts End Point Simple Definition Chemistry The completion of a titration is the end point, detected by some type of physical change produced by the solution, such as a color change. Using a ph probe and using a colour indicator. The closer the end point to the equivalence point the better, but it is often not easy to find a good method of. The point during. End Point Simple Definition Chemistry.
From www.reddit.com
We reached the end point after 25 years r/pokemonanime End Point Simple Definition Chemistry The end point is where the titration ends in practice. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The main difference between an equivalence point and an endpoint is that the former marks the end of the reaction whereas. The point during a titration when an indicator shows that. End Point Simple Definition Chemistry.
From www.independent.ie
Lee Carsley back in the frame as FAI confirm Ireland manager search ‘at End Point Simple Definition Chemistry Using a ph probe and using a colour indicator. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The end point is where the titration ends in practice. For a ph titration (between an acid and a base), there are two common ways to find. End Point Simple Definition Chemistry.
From ar.inspiredpencil.com
Endpoint Chemistry End Point Simple Definition Chemistry The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Titrations with a ph probe. The end point is where the titration ends in practice. Using a ph probe and using a colour indicator. The completion of a titration is the end point, detected by some type of physical. End Point Simple Definition Chemistry.