Magnesium Bromide Anion . Write the symbol for each ion and. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Write the symbol for each ion and. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number of electrons required to fill the valence. Predict the charge of monatomic main group elements based on their group number. The magnesium ion has a 2+, so it requires 2 bromide anions, each with a single negative charge, to balance the 2 positive charges of magnesium. Magnesium and nitrogen react to form an ionic compound. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Magnesium and nitrogen react to form an ionic compound. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. So the formula of the. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion.
from www.numerade.com
Predict which forms an anion, which forms a cation, and the charges of each ion. All the anions are of this type, gaining the number of electrons required to fill the valence. Magnesium and nitrogen react to form an ionic compound. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the symbol for each ion and. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Magnesium and nitrogen react to form an ionic compound. Predict the charge of monatomic main group elements based on their group number.
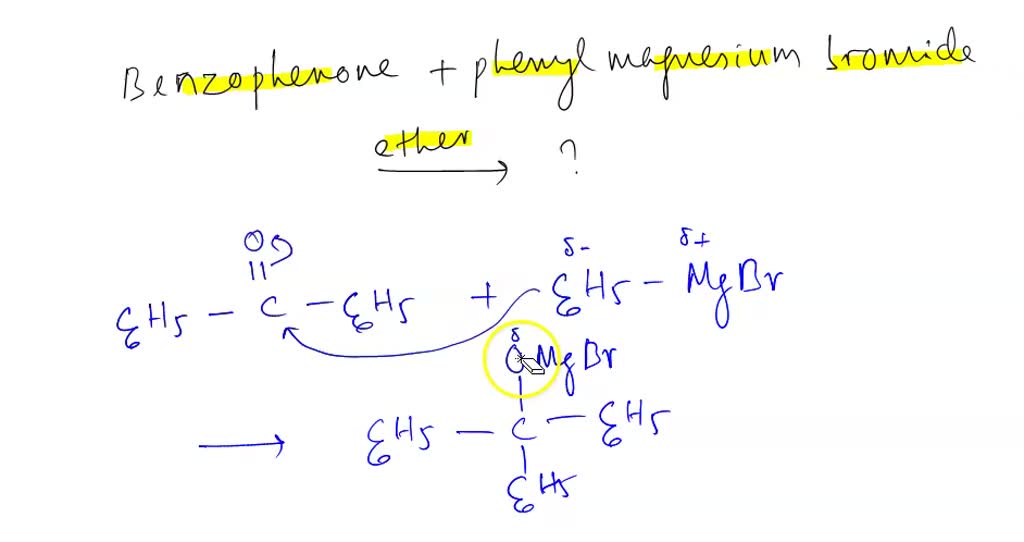
SOLVED From Grignard reagent phenyl magnesium bromide After the
Magnesium Bromide Anion Magnesium and nitrogen react to form an ionic compound. All the anions are of this type, gaining the number of electrons required to fill the valence. So the formula of the. The magnesium ion has a 2+, so it requires 2 bromide anions, each with a single negative charge, to balance the 2 positive charges of magnesium. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Write the symbol for each ion and. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the symbol for each ion and. Magnesium and nitrogen react to form an ionic compound. Magnesium and nitrogen react to form an ionic compound. Predict the charge of monatomic main group elements based on their group number.
From www.numerade.com
SOLVED Predict the products of the following reactions.(a) allyl Magnesium Bromide Anion Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Magnesium and nitrogen react to form an ionic compound. The magnesium ion has a 2+, so it requires 2 bromide anions, each with a single negative charge, to balance the 2 positive charges of magnesium. Figure 2.7.2 lists the ions (cation and anion). Magnesium Bromide Anion.
From www.biosynth.com
FA35367 1730252 Allyl magnesium bromide solution Magnesium Bromide Anion Predict the charge of monatomic main group elements based on their group number. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each. Magnesium Bromide Anion.
From www.youtube.com
Draw the Lewis Structure of MgBr2 (magnesium bromide) YouTube Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Magnesium and nitrogen react to form an ionic compound. The magnesium ion has a 2+, so it requires 2 bromide anions, each with a single negative charge, to. Magnesium Bromide Anion.
From jiji.ng
Magnesium Bromide 100g in Lagos Island (Eko) Medical Supplies Magnesium Bromide Anion All the anions are of this type, gaining the number of electrons required to fill the valence. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Write the symbol. Magnesium Bromide Anion.
From chemistry291.blogspot.com
Magnesium Bromide FormulaFormula for Magnesium Bromide Magnesium Bromide Anion Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict the charge of monatomic main group elements based on their group number. So the formula of the. The magnesium ion has a 2+, so it requires 2 bromide anions, each with. Magnesium Bromide Anion.
From www.smolecule.com
Buy Magnesium bromide (MgBr2) 7789482 Magnesium Bromide Anion Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and. Write the symbol for each ion and. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium bromide is a white. Magnesium Bromide Anion.
From www.fishersci.com
Magnesium bromide ethyl etherate, 99, ACROS Organics Fisher Scientific Magnesium Bromide Anion Write the symbol for each ion and. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. All the anions are of. Magnesium Bromide Anion.
From edurev.in
CO₂ on reaction with ethyl magnesium bromide givesa)Ethaneb)Propanoic Magnesium Bromide Anion Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. So the formula of the. Magnesium and nitrogen react to form an ionic compound. Write the symbol for. Magnesium Bromide Anion.
From cabinet.matttroy.net
Properties Of Magnesium On The Periodic Table Matttroy Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and. All the anions are of this type, gaining the number of electrons required to fill the valence. Predict the charge of monatomic main group elements based on their group number. Magnesium bromide is a white crystalline ionic compound. Magnesium Bromide Anion.
From www.fishersci.com
[4(1Pyrrolidinylmethyl)phenyl]magnesium bromide, 0.25M solution in Magnesium Bromide Anion Write the symbol for each ion and. Write the symbol for each ion and. Magnesium and nitrogen react to form an ionic compound. Predict the charge of monatomic main group elements based on their group number. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. The magnesium ion has a 2+, so it. Magnesium Bromide Anion.
From askfilo.com
Mg Mg2++2e−2,8,22,8 (Magnesium cation) (Chloride anion) Figure 3.6 For.. Magnesium Bromide Anion So the formula of the. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium and nitrogen react to form an ionic compound. Magnesium and nitrogen react to form an ionic compound. Predict the charge of monatomic main group elements based on their group number. The magnesium ion has a 2+, so it requires 2 bromide. Magnesium Bromide Anion.
From askfilo.com
(4.) CO2 on reaction with ethyl magnesium bromide followed by protonatio.. Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. So the formula of the. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Magnesium and nitrogen react to form an ionic compound. All the anions are of this type, gaining the number of electrons required to fill the valence. The. Magnesium Bromide Anion.
From byjus.com
Methyl cyanide on treatment with magnesium bromide followed by Magnesium Bromide Anion Write the symbol for each ion and. Magnesium and nitrogen react to form an ionic compound. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. All the anions are. Magnesium Bromide Anion.
From synthonix.com
Synthonix, Inc > Grignards and Zincs > 620607241 (1,2 Magnesium Bromide Anion Magnesium and nitrogen react to form an ionic compound. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. All the anions are of this type, gaining the number of electrons required to fill the valence. The magnesium ion has a 2+, so it requires 2 bromide anions, each with a single negative charge,. Magnesium Bromide Anion.
From www.numerade.com
SOLVED benzyl magnesium bromide on reaction with acetone followed by Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict the charge of monatomic main group elements based on their group number. Magnesium. Magnesium Bromide Anion.
From www.fishersci.ca
Magnesium bromide, 98, pure, anhydrous, Thermo Scientific™ Fisher Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Predict the charge of monatomic main group elements based on their group number. So the formula of the. Magnesium and nitrogen react to form an ionic compound.. Magnesium Bromide Anion.
From www.biosynth.com
FM165013 75161 Methyl magnesium bromide 1M in THF Magnesium Bromide Anion Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and. So the formula of the. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form. Magnesium Bromide Anion.
From www.numerade.com
SOLVEDPhenyl magnesium bromide reacts with methanol to give (a) a Magnesium Bromide Anion Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium. Magnesium Bromide Anion.
From slideplayer.com
Chemical Nomenclature ppt download Magnesium Bromide Anion Magnesium and nitrogen react to form an ionic compound. Predict the charge of monatomic main group elements based on their group number. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Write the symbol for each ion and. So the formula of the. Write formulas for ionic compounds using monatomic and polyatomic ions. Magnesium Bromide Anion.
From byjus.com
When phenyl magnesium bromide reacts with t bu†an ol,the product would b Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. All the anions are of this type, gaining the number of electrons required to fill the valence. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Write the symbol for each ion and. Magnesium and nitrogen react. Magnesium Bromide Anion.
From www.nanochemazone.com
Magnesium Bromide Powder Low Price 40 Highly pure Nanochemazone Magnesium Bromide Anion Predict the charge of monatomic main group elements based on their group number. All the anions are of this type, gaining the number of electrons required to fill the valence. Predict which forms an anion, which forms a cation, and the charges of each ion. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form. Magnesium Bromide Anion.
From www.numerade.com
SOLVED Magnesium bromide is a binary ionic compound. From its formula Magnesium Bromide Anion Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the symbol for each ion and. Predict the charge of monatomic main group elements based on their group number. All the anions are of this type, gaining the number of electrons required to fill the valence. The magnesium ion has a 2+, so it requires 2. Magnesium Bromide Anion.
From www.toppr.com
What happens when methyl magnesium bromide is treated with formaldehyde Magnesium Bromide Anion Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and. All the anions are of this type, gaining the number of electrons required to fill the valence. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. So the formula of the. Magnesium bromide is a white crystalline. Magnesium Bromide Anion.
From www.numerade.com
SOLVED From Grignard reagent phenyl magnesium bromide After the Magnesium Bromide Anion Predict the charge of monatomic main group elements based on their group number. Write the symbol for each ion and. All the anions are of this type, gaining the number of electrons required to fill the valence. Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and. Magnesium and nitrogen react to form an. Magnesium Bromide Anion.
From www.numerade.com
SOLVED Text Determine the Formula and Molar Mass of each of the Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. All the anions are of this type, gaining the number of electrons required to fill the valence. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation. Magnesium Bromide Anion.
From www.fishersci.com
Fisher Science Education Magnesium Bromide Hexahydrate Fisher Scientific Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. Write the symbol for each ion and. Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Magnesium and nitrogen react to form an ionic compound. Write formulas for ionic compounds using monatomic and polyatomic ions by. Magnesium Bromide Anion.
From www.biosynth.com
FN35187 693254 nPentylmagnesium bromide 2.0 M in diethyl ether Magnesium Bromide Anion Write the symbol for each ion and. The magnesium ion has a 2+, so it requires 2 bromide anions, each with a single negative charge, to balance the 2 positive charges of magnesium. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Predict which forms an anion, which forms a cation, and. Magnesium Bromide Anion.
From www.calpaclab.com
Magnesium bromide, anhydrous, 25 grams Magnesium Bromide Anion Write the symbol for each ion and. All the anions are of this type, gaining the number of electrons required to fill the valence. So the formula of the. Predict the charge of monatomic main group elements based on their group number. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Write formulas. Magnesium Bromide Anion.
From www.youtube.com
Is MgBr2 (Magnesium bromide) Ionic or Covalent? YouTube Magnesium Bromide Anion Predict which forms an anion, which forms a cation, and the charges of each ion. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Predict the charge of monatomic main group elements based on their group number. Magnesium and nitrogen react to form an ionic compound. The magnesium ion has a 2+,. Magnesium Bromide Anion.
From byjus.com
Phenyl magnesium bromide reacts with methanol to give Magnesium Bromide Anion Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. So the formula of the. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. All the anions are of this type, gaining the number of electrons required to. Magnesium Bromide Anion.
From synthonix.com
Synthonix, Inc > Grignards and Zincs > 2128716334 3Tetrahydrofuran Magnesium Bromide Anion All the anions are of this type, gaining the number of electrons required to fill the valence. So the formula of the. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each. Magnesium Bromide Anion.
From www.toppr.com
What happens when formaldehyde is treated with methyl magnesium bromide Magnesium Bromide Anion Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. All the anions are of this type, gaining the number of electrons required to fill the valence. The magnesium ion. Magnesium Bromide Anion.
From www.doubtnut.com
Which compound on reaction with ethyl magnesium bromide and water will Magnesium Bromide Anion Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge neutrality. Predict which forms an anion, which forms a cation, and the charges of each ion. Predict which forms an anion, which forms a cation, and. Magnesium Bromide Anion.
From www.youtube.com
How to Draw the Lewis Dot Structure for MgBr2 Magnesium bromide YouTube Magnesium Bromide Anion Magnesium bromide is a white crystalline ionic compound with a chemical formula mgbr2 m g b r 2. The magnesium ion has a 2+, so it requires 2 bromide anions, each with a single negative charge, to balance the 2 positive charges of magnesium. Write formulas for ionic compounds using monatomic and polyatomic ions by applying the principle of charge. Magnesium Bromide Anion.
From alchetron.com
Magnesium bromide Alchetron, The Free Social Encyclopedia Magnesium Bromide Anion Write the symbol for each ion and. So the formula of the. Recognize that metals lose electrons to form cations and that nonmetals gain electrons to form anions. Magnesium and nitrogen react to form an ionic compound. Predict the charge of monatomic main group elements based on their group number. Predict which forms an anion, which forms a cation, and. Magnesium Bromide Anion.