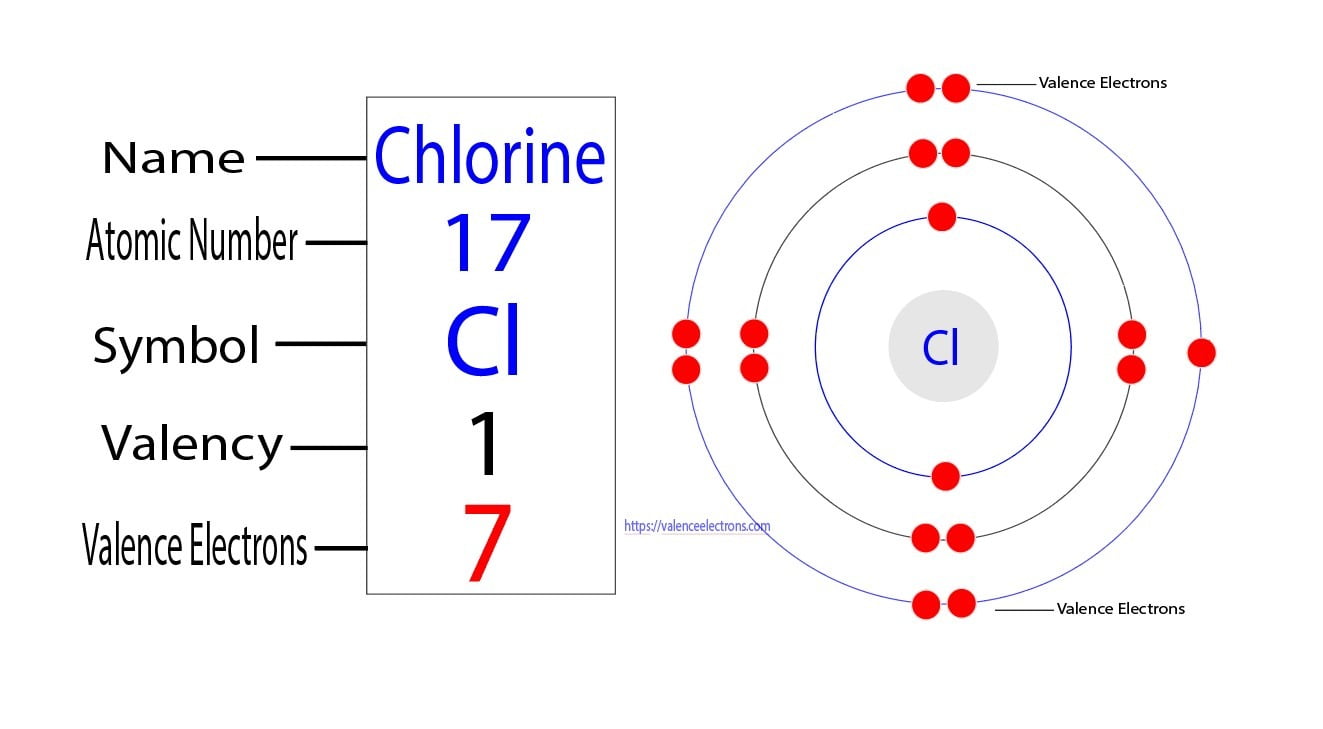
How Many Energy Levels Does An Atom Of Chlorine Have . from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. Ionisation energies and electron affinity. The electron affinity of chlorine is 349.0 kj mol ‑1. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : a representation of the atomic spectrum of chlorine.
from www.animalia-life.club
Ionisation energies and electron affinity. The electron affinity of chlorine is 349.0 kj mol ‑1. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. a representation of the atomic spectrum of chlorine. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration :
Chloride Ion Number Of Protons And Electrons
How Many Energy Levels Does An Atom Of Chlorine Have chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. The electron affinity of chlorine is 349.0 kj mol ‑1. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. a representation of the atomic spectrum of chlorine. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. Ionisation energies and electron affinity.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons How Many Energy Levels Does An Atom Of Chlorine Have The electron affinity of chlorine is 349.0 kj mol ‑1. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. a representation of the atomic spectrum of chlorine. these orbits form electron shells or. How Many Energy Levels Does An Atom Of Chlorine Have.
From circuitlistethiops123.z13.web.core.windows.net
Atomic Diagram Of Chlorine How Many Energy Levels Does An Atom Of Chlorine Have Ionisation energies and electron affinity. The electron affinity of chlorine is 349.0 kj mol ‑1. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. a representation of the atomic spectrum of chlorine. chlorine is the 17th element in the periodic table and has a. How Many Energy Levels Does An Atom Of Chlorine Have.
From mavink.com
Periodic Table With Energy Levels How Many Energy Levels Does An Atom Of Chlorine Have Ionisation energies and electron affinity. a representation of the atomic spectrum of chlorine. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. chlorine is. How Many Energy Levels Does An Atom Of Chlorine Have.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram How Many Energy Levels Does An Atom Of Chlorine Have from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.dreamstime.com
An Atom of Chlorine Diagram Stock Vector Illustration of structure How Many Energy Levels Does An Atom Of Chlorine Have a representation of the atomic spectrum of chlorine. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. Ionisation energies and electron affinity. The electron affinity of chlorine is 349.0 kj. How Many Energy Levels Does An Atom Of Chlorine Have.
From hubpages.com
Atoms and Atomic Structure HubPages How Many Energy Levels Does An Atom Of Chlorine Have chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : The electron affinity of chlorine is 349.0 kj mol ‑1. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. a representation of the atomic spectrum of chlorine. chlorine is the 17th element in. How Many Energy Levels Does An Atom Of Chlorine Have.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements How Many Energy Levels Does An Atom Of Chlorine Have these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. The electron affinity of chlorine is 349.0 kj mol ‑1. Ionisation energies and electron affinity. chlorine (cl) energy. How Many Energy Levels Does An Atom Of Chlorine Have.
From diagramweb.net
Molecular Orbital Diagram For Cl2 How Many Energy Levels Does An Atom Of Chlorine Have The electron affinity of chlorine is 349.0 kj mol ‑1. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : Ionisation energies and electron affinity. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.online-sciences.com
Atomic structure of matter, Energy levels, Electronic distribution and How Many Energy Levels Does An Atom Of Chlorine Have these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. a representation of the atomic spectrum of chlorine. Ionisation energies and electron affinity. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : from the electron configuration of neutral chlorine atoms. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library How Many Energy Levels Does An Atom Of Chlorine Have these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. Ionisation energies and electron affinity. The electron affinity of chlorine is 349.0 kj mol ‑1. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : from the electron configuration of neutral chlorine. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.expii.com
Ions — Definition & Overview Expii How Many Energy Levels Does An Atom Of Chlorine Have The electron affinity of chlorine is 349.0 kj mol ‑1. a representation of the atomic spectrum of chlorine. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. chlorine is the. How Many Energy Levels Does An Atom Of Chlorine Have.
From wirepartnemertines.z5.web.core.windows.net
Diagram Of Chlorine Atom How Many Energy Levels Does An Atom Of Chlorine Have chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. a representation of the atomic spectrum of chlorine. The electron affinity of chlorine is 349.0 kj mol ‑1. Ionisation energies and electron affinity. these orbits form electron shells or energy levels, which are a way of visualizing the number of. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons How Many Energy Levels Does An Atom Of Chlorine Have chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : a representation of the atomic spectrum of chlorine. Ionisation energies and electron affinity. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine is the 17th element in the periodic table and has. How Many Energy Levels Does An Atom Of Chlorine Have.
From wirepartnemertines.z5.web.core.windows.net
Diagram Of Chlorine Atom How Many Energy Levels Does An Atom Of Chlorine Have chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. a representation of the atomic spectrum of chlorine. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. The electron affinity of chlorine is 349.0 kj mol ‑1.. How Many Energy Levels Does An Atom Of Chlorine Have.
From chloebarrett.z13.web.core.windows.net
Electron Energy Level Chart How Many Energy Levels Does An Atom Of Chlorine Have chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. The electron affinity of chlorine is 349.0 kj mol ‑1. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many. How Many Energy Levels Does An Atom Of Chlorine Have.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements How Many Energy Levels Does An Atom Of Chlorine Have Ionisation energies and electron affinity. The electron affinity of chlorine is 349.0 kj mol ‑1. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.slideserve.com
PPT 3 rd Orbitals PowerPoint Presentation, free download ID2062267 How Many Energy Levels Does An Atom Of Chlorine Have The electron affinity of chlorine is 349.0 kj mol ‑1. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. chlorine (cl) energy levels of neutral chlorine ( cl i ). How Many Energy Levels Does An Atom Of Chlorine Have.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons How Many Energy Levels Does An Atom Of Chlorine Have Ionisation energies and electron affinity. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. a representation of the atomic spectrum of chlorine. chlorine (cl) energy levels of neutral chlorine. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.dreamstime.com
Chlorine Atom, with Mass and Energy Levels. Stock Vector Illustration How Many Energy Levels Does An Atom Of Chlorine Have these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : Ionisation energies and. How Many Energy Levels Does An Atom Of Chlorine Have.
From basichemistry.blogspot.com
Basic Chemistry October 2012 How Many Energy Levels Does An Atom Of Chlorine Have chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. The electron affinity of chlorine is 349.0 kj mol ‑1. Ionisation energies and electron affinity. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. a representation of. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.shutterstock.com
585 Chlorine electrons 이미지, 스톡 사진 및 벡터 Shutterstock How Many Energy Levels Does An Atom Of Chlorine Have from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. a representation of the atomic spectrum of chlorine. chlorine (cl) energy levels of neutral chlorine. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.slideserve.com
PPT ATOMIC THEORY REVIEW PowerPoint Presentation, free download ID How Many Energy Levels Does An Atom Of Chlorine Have chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core. How Many Energy Levels Does An Atom Of Chlorine Have.
From material-properties.org
Chlorine Periodic Table and Atomic Properties How Many Energy Levels Does An Atom Of Chlorine Have from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. a representation of the atomic spectrum of chlorine. chlorine (cl) energy levels of neutral chlorine. How Many Energy Levels Does An Atom Of Chlorine Have.
From stock.adobe.com
Periodic Table of the Elements, Shell Structure of Chlorine Cl How Many Energy Levels Does An Atom Of Chlorine Have Ionisation energies and electron affinity. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. The electron affinity of chlorine is 349.0 kj mol ‑1. from the electron configuration of neutral chlorine. How Many Energy Levels Does An Atom Of Chlorine Have.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille How Many Energy Levels Does An Atom Of Chlorine Have a representation of the atomic spectrum of chlorine. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine (cl) energy levels of neutral chlorine. How Many Energy Levels Does An Atom Of Chlorine Have.
From slideplayer.com
ATOMS Standard C2 Students will demonstrate an understanding of atomic How Many Energy Levels Does An Atom Of Chlorine Have chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : Ionisation energies and electron affinity. The electron affinity of chlorine is 349.0 kj mol ‑1. a representation of the atomic spectrum of chlorine. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost. How Many Energy Levels Does An Atom Of Chlorine Have.
From circuitetappesu.z4.web.core.windows.net
Atomic Diagram Of Chlorine How Many Energy Levels Does An Atom Of Chlorine Have The electron affinity of chlorine is 349.0 kj mol ‑1. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. Ionisation energies and electron affinity. a representation of the atomic spectrum of chlorine. these orbits form electron shells or energy levels, which are a way of visualizing the. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science How Many Energy Levels Does An Atom Of Chlorine Have Ionisation energies and electron affinity. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. a representation of the atomic spectrum of chlorine. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : chlorine is the 17th element in the periodic. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.numerade.com
SOLVEDA chlorine atom in its ground state has a total of seven How Many Energy Levels Does An Atom Of Chlorine Have Ionisation energies and electron affinity. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : The electron affinity of chlorine is 349.0 kj mol ‑1. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many. How Many Energy Levels Does An Atom Of Chlorine Have.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun How Many Energy Levels Does An Atom Of Chlorine Have Ionisation energies and electron affinity. The electron affinity of chlorine is 349.0 kj mol ‑1. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. a representation of the atomic spectrum. How Many Energy Levels Does An Atom Of Chlorine Have.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? How Many Energy Levels Does An Atom Of Chlorine Have chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : The electron affinity of chlorine is 349.0 kj mol ‑1. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many. How Many Energy Levels Does An Atom Of Chlorine Have.
From dxoatswvu.blob.core.windows.net
Chlorine Valence Atom at Connie Downs blog How Many Energy Levels Does An Atom Of Chlorine Have a representation of the atomic spectrum of chlorine. from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : these orbits form electron shells or energy levels, which are a way of visualizing the number. How Many Energy Levels Does An Atom Of Chlorine Have.
From profliway.hubpages.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind How Many Energy Levels Does An Atom Of Chlorine Have these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. The electron affinity of chlorine is 349.0 kj mol ‑1. Ionisation energies and electron affinity. a representation of the atomic spectrum of chlorine. chlorine is the 17th element in the periodic table and has a. How Many Energy Levels Does An Atom Of Chlorine Have.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons How Many Energy Levels Does An Atom Of Chlorine Have from the electron configuration of neutral chlorine atoms (exercise \(\pageindex{1}\)), how many valence electrons and how many core electrons. chlorine is the 17th element in the periodic table and has a symbol of cl and atomic. these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost. How Many Energy Levels Does An Atom Of Chlorine Have.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms How Many Energy Levels Does An Atom Of Chlorine Have these orbits form electron shells or energy levels, which are a way of visualizing the number of electrons in the outermost shells. a representation of the atomic spectrum of chlorine. chlorine (cl) energy levels of neutral chlorine ( cl i ) configuration : The electron affinity of chlorine is 349.0 kj mol ‑1. chlorine is the. How Many Energy Levels Does An Atom Of Chlorine Have.