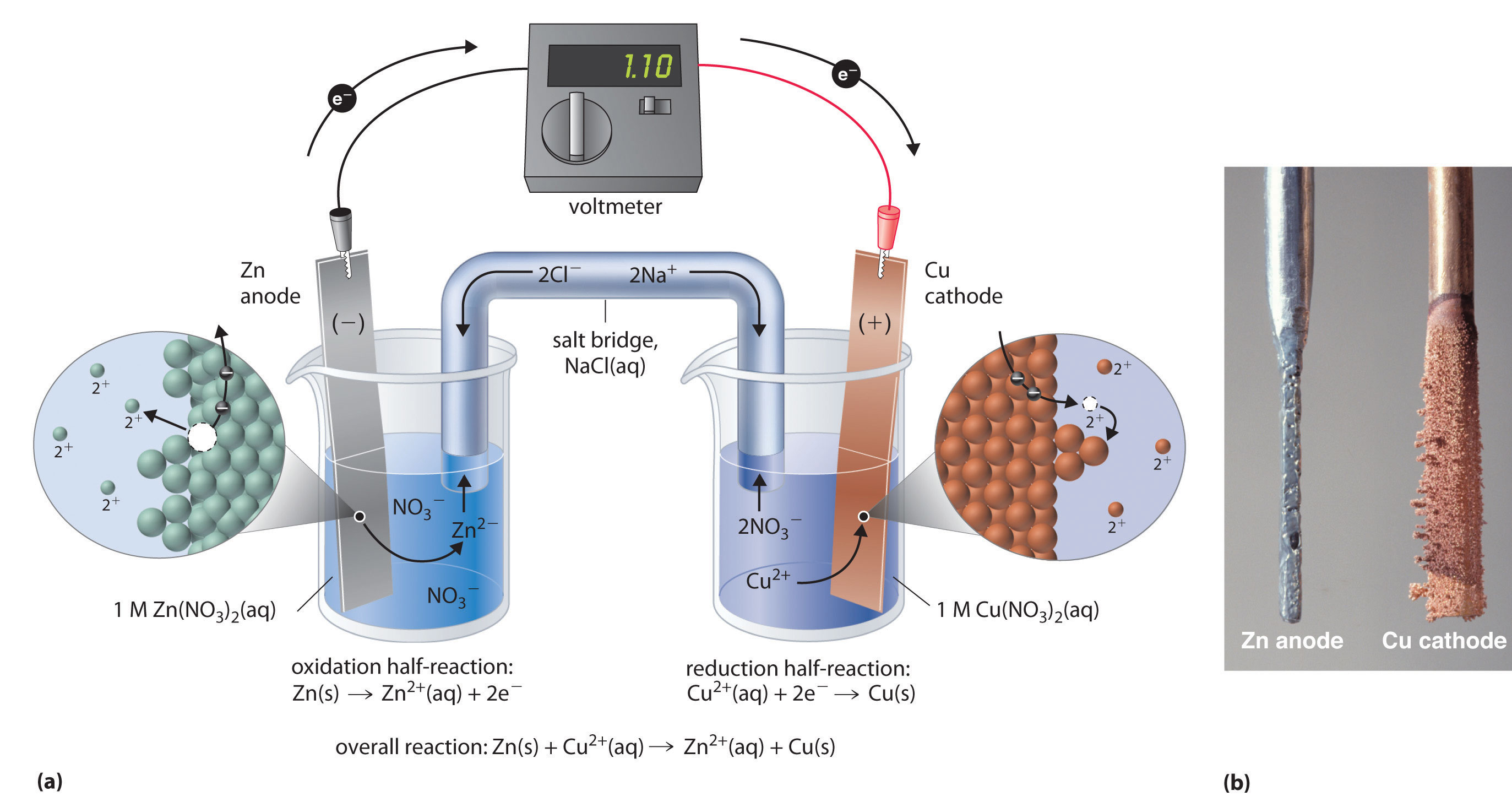
What Is The Emf Of A Galvanic Cell . the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place.
from 2012books.lardbucket.org
the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the.
Describing Electrochemical Cells
What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic.
From www.chemicals.co.uk
Measuring The EMF Of An Electrochemical Cell The Chemistry Blog What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. additionally,. What Is The Emf Of A Galvanic Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the. What Is The Emf Of A Galvanic Cell.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively,. What Is The Emf Of A Galvanic Cell.
From dxodcnqen.blob.core.windows.net
Standard Hydrogen Electrode In A Galvanic Cell at Katharine Murphy blog What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively,. What Is The Emf Of A Galvanic Cell.
From thechemistrynotes.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle What Is The Emf Of A Galvanic Cell a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause. What Is The Emf Of A Galvanic Cell.
From www.pw.live
EMF Of A Cell, Definition, Formula, Types, And Methods What Is The Emf Of A Galvanic Cell additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell or voltaic cell, named after the scientists luigi galvani and. What Is The Emf Of A Galvanic Cell.
From www.youtube.com
Introduction to Galvanic Cells & Voltaic Cells YouTube What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and. What Is The Emf Of A Galvanic Cell.
From www.pinterest.com
What Is a Galvanic Cell? Galvanic cell, Electrochemical cell, Science What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons. What Is The Emf Of A Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the emf of such a cell is said to be its standard electromotive force and is given. What Is The Emf Of A Galvanic Cell.
From www.alamy.com
Simple electrochemical or galvanic cell. The Daniell cell Stock Photo What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. additionally,. What Is The Emf Of A Galvanic Cell.
From www.youtube.com
Galvanic cells and EMF YouTube What Is The Emf Of A Galvanic Cell additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the emf of such a cell is said to be its standard electromotive. What Is The Emf Of A Galvanic Cell.
From brainly.in
What is the emf of a galvanic cell consisting of a Cd2+/Cd halfcell What Is The Emf Of A Galvanic Cell additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. devices of this sort are generally referred to as electrochemical cells, and those in which. What Is The Emf Of A Galvanic Cell.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. the emf of such a cell is said to be its standard electromotive force and is given the symbol. What Is The Emf Of A Galvanic Cell.
From saylordotorg.github.io
Electrochemistry What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. . What Is The Emf Of A Galvanic Cell.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle What Is The Emf Of A Galvanic Cell a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the. What Is The Emf Of A Galvanic Cell.
From www.youtube.com
Emf of daniel cell from nernst equation(Electrochemistry part 28 for What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. additionally,. What Is The Emf Of A Galvanic Cell.
From www.youtube.com
Galvanic Cell Example Daniell Cell YouTube What Is The Emf Of A Galvanic Cell additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. devices of this sort are generally referred to as electrochemical cells, and those in. What Is The Emf Of A Galvanic Cell.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. the emf of such a cell is said to be its standard electromotive force and is given the symbol. What Is The Emf Of A Galvanic Cell.
From chemistry.stackexchange.com
physical chemistry Finding EMF of a galvanic cell without standard What Is The Emf Of A Galvanic Cell a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. the emf of such a cell is said to be its standard electromotive force and. What Is The Emf Of A Galvanic Cell.
From exomvxjlq.blob.core.windows.net
The Galvanic Cell Described By at Geraldo Walls blog What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. . What Is The Emf Of A Galvanic Cell.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 110 What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the.. What Is The Emf Of A Galvanic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry What Is The Emf Of A Galvanic Cell a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the emf of such a cell is said to be its standard electromotive force and is given. What Is The Emf Of A Galvanic Cell.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the.. What Is The Emf Of A Galvanic Cell.
From biochemreview.weebly.com
Galvanic cell BIOChemReview What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons. What Is The Emf Of A Galvanic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. . What Is The Emf Of A Galvanic Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and What Is The Emf Of A Galvanic Cell devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the. What Is The Emf Of A Galvanic Cell.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER What Is The Emf Of A Galvanic Cell the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the. What Is The Emf Of A Galvanic Cell.
From userdatarheumatics.z21.web.core.windows.net
Simple Cell Diagram Chemistry What Is The Emf Of A Galvanic Cell the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro. What Is The Emf Of A Galvanic Cell.
From www.chegg.com
Solved What is the standard emf of a galvanic cell made of a What Is The Emf Of A Galvanic Cell the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively,. What Is The Emf Of A Galvanic Cell.
From mmerevise.co.uk
Calculating the EMF What Is The Emf Of A Galvanic Cell the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons. What Is The Emf Of A Galvanic Cell.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram What Is The Emf Of A Galvanic Cell a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. devices of. What Is The Emf Of A Galvanic Cell.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC What Is The Emf Of A Galvanic Cell additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the emf of such a cell is said to be its standard electromotive. What Is The Emf Of A Galvanic Cell.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts What Is The Emf Of A Galvanic Cell the emf of such a cell is said to be its standard electromotive force and is given the symbol e°. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. devices of. What Is The Emf Of A Galvanic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working What Is The Emf Of A Galvanic Cell additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. devices of this sort are generally referred to as electrochemical cells, and those in which a spontaneous redox reaction takes place. the electromotive force (emf) is the maximum potential difference between two electrodes of. What Is The Emf Of A Galvanic Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells What Is The Emf Of A Galvanic Cell additionally, the electromotive force of a galvanic cell specifies the ability of an electrochemical cell to cause the flow of electrons through the. a galvanic cell or voltaic cell, named after the scientists luigi galvani and alessandro volta, respectively, is an electrochemical. the electromotive force (emf) is the maximum potential difference between two electrodes of a galvanic.. What Is The Emf Of A Galvanic Cell.