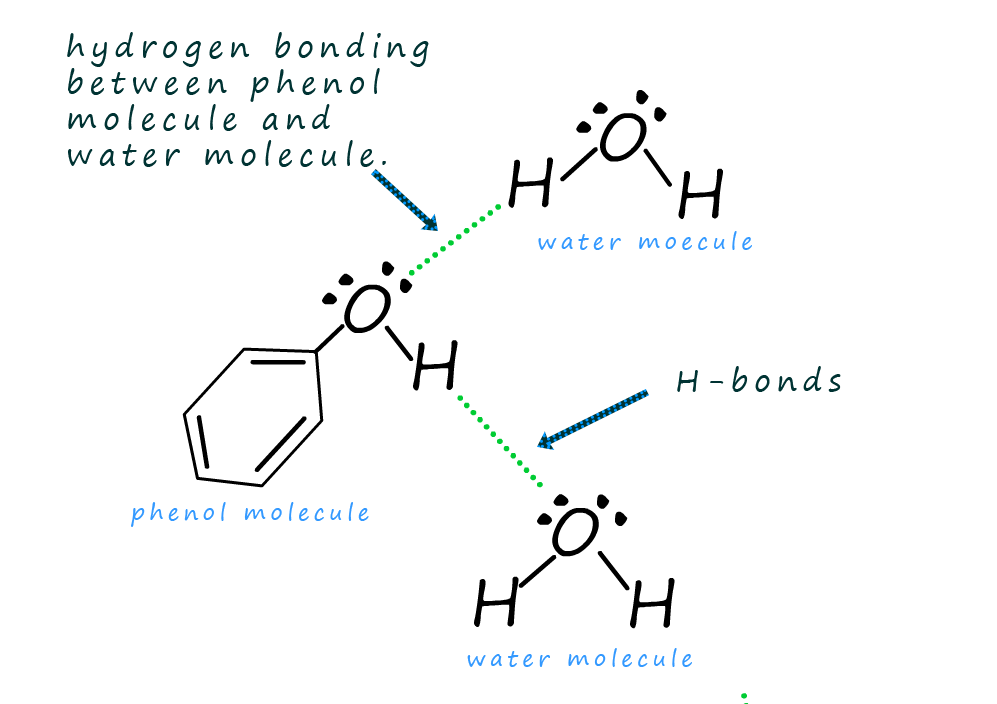
What Are Phenols Soluble In Water . the solubility of phenol in water is governed by the hydroxyl group present. Phenol is partially soluble in water. small alcohols are completely soluble in water; phenols are similar to alcohols but form stronger hydrogen bonds. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. If you try to dissolve more than this, you get two layers of liquid. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. Phenol acts as a weak acid. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. However, it is more soluble in organic solvents like ether, acetone, and chloroform. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. Thus, they are more soluble in water than are alcohols. If you try to dissolve more than this, you get two. Is phenol an acid or base. Mixing the two in any proportion generates a single solution.
from www.science-revision.co.uk
the solubility of phenol in water is governed by the hydroxyl group present. phenols are similar to alcohols but form stronger hydrogen bonds. Thus, they are more soluble in water than are alcohols. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. Is phenol an acid or base. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. small alcohols are completely soluble in water; Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. If you try to dissolve more than this, you get two.
Phenolproperties
What Are Phenols Soluble In Water Mixing the two in any proportion generates a single solution. Phenol is partially soluble in water. the solubility of phenol in water is governed by the hydroxyl group present. Thus, they are more soluble in water than are alcohols. Is phenol an acid or base. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. Mixing the two in any proportion generates a single solution. If you try to dissolve more than this, you get two. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. small alcohols are completely soluble in water; If you try to dissolve more than this, you get two layers of liquid. phenols are similar to alcohols but form stronger hydrogen bonds. Phenol acts as a weak acid. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. However, it is more soluble in organic solvents like ether, acetone, and chloroform.
From slideplayer.com
Fundamentals of Organic Chemistry ppt download What Are Phenols Soluble In Water Thus, they are more soluble in water than are alcohols. Mixing the two in any proportion generates a single solution. phenols are similar to alcohols but form stronger hydrogen bonds. If you try to dissolve more than this, you get two. Phenol acts as a weak acid. Thus, hydrogen bonds are formed between water and phenol molecules which make. What Are Phenols Soluble In Water.
From www.sciencephoto.com
Phenol reactions Stock Image A500/0274 Science Photo Library What Are Phenols Soluble In Water Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. Phenol is partially soluble in water. If you try to dissolve more than this, you get two. Phenol acts as a weak acid. If you try to dissolve more than this, you get two layers of liquid. phenols are similar to alcohols but. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT PHENOLS PowerPoint Presentation, free download ID9445745 What Are Phenols Soluble In Water Thus, they are more soluble in water than are alcohols. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. However, it is more soluble in organic solvents like ether, acetone, and chloroform. Mixing the two in any proportion generates a single solution. small alcohols are completely soluble in water; Thus, hydrogen. What Are Phenols Soluble In Water.
From kadence-yersbloghamilton.blogspot.com
Is Phenol Soluble in Water What Are Phenols Soluble In Water If you try to dissolve more than this, you get two layers of liquid. However, it is more soluble in organic solvents like ether, acetone, and chloroform. Is phenol an acid or base. Phenol is partially soluble in water. Thus, they are more soluble in water than are alcohols. If you try to dissolve more than this, you get two.. What Are Phenols Soluble In Water.
From studylib.net
Phenols — ArOH What Are Phenols Soluble In Water Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. Phenol is partially soluble in water. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. Is phenol an. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT PHENOLS PowerPoint Presentation, free download ID9445745 What Are Phenols Soluble In Water Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. Phenol acts as a weak acid. Thus, they are more soluble in water than are alcohols. The hydroxyl group can release a proton (h +) from the oxygen atom,. What Are Phenols Soluble In Water.
From www.chegg.com
Solved ALCOHOLS, PHENOLS & CARBOXYLIC ACIDS 1. Solubility in What Are Phenols Soluble In Water The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. If you try to dissolve more than this, you get two layers of liquid. If you try to dissolve more than this, you get two. phenols are similar to alcohols but form stronger hydrogen bonds. Phenol acts as a weak acid. Thus, hydrogen bonds are. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and What Are Phenols Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. Phenol acts as a weak acid. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. Thus, hydrogen bonds are formed between. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT PHENOLS PowerPoint Presentation, free download ID9445745 What Are Phenols Soluble In Water Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. small alcohols are completely soluble in water; If you try to dissolve more than this, you get two. the solubility of phenol in water is governed by the hydroxyl group present. However, it is more soluble in organic solvents like ether,. What Are Phenols Soluble In Water.
From www.researchgate.net
Watersoluble phenols (WSP) content in the soil at different What Are Phenols Soluble In Water Phenol acts as a weak acid. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more than this, you get two layers of liquid. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. phenols are similar to alcohols but form stronger hydrogen. What Are Phenols Soluble In Water.
From www.youtube.com
Phenol and Sodium Hydroxide Reaction C6H5OH + NaOH pH Value Heat What Are Phenols Soluble In Water The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. phenols are similar to alcohols but form stronger hydrogen bonds. Thus, they are more soluble in water than are alcohols. Is phenol an acid or base. If you try to dissolve more than this, you get two layers of. What Are Phenols Soluble In Water.
From www.semanticscholar.org
Solubility of Phenolic Compounds in Pure Water and Alcohols with FTIR What Are Phenols Soluble In Water Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. Phenol is partially soluble in water. Is phenol an acid or base. The hydroxyl group in phenol is involved in the formation of. What Are Phenols Soluble In Water.
From present5.com
Chapter 7 Aromatic Compounds Table of Contents 1 What Are Phenols Soluble In Water Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. However, it is more soluble in organic solvents like ether, acetone, and chloroform. Phenol is partially soluble in water. Mixing the two in any proportion generates a. What Are Phenols Soluble In Water.
From gbu-presnenskij.ru
Water Phenol PDF Solubility Phase (Matter), 58 OFF What Are Phenols Soluble In Water Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more than this, you get two. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. If you try to. What Are Phenols Soluble In Water.
From www.science-revision.co.uk
Phenolproperties What Are Phenols Soluble In Water Phenol is partially soluble in water. Mixing the two in any proportion generates a single solution. small alcohols are completely soluble in water; Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. Phenol acts as a weak acid. phenols are similar to alcohols but form stronger hydrogen bonds. If you try. What Are Phenols Soluble In Water.
From www.youtube.com
PhenolWater System, Chemistry Lecture Sabaq.pk YouTube What Are Phenols Soluble In Water phenols are similar to alcohols but form stronger hydrogen bonds. the solubility of phenol in water is governed by the hydroxyl group present. However, it is more soluble in organic solvents like ether, acetone, and chloroform. Phenol acts as a weak acid. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. Phenol is. What Are Phenols Soluble In Water.
From peterkruwwatson.blogspot.com
Is Phenol Soluble in Water PeterkruwWatson What Are Phenols Soluble In Water If you try to dissolve more than this, you get two. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. Phenol is partially soluble in water. the solubility of phenol in water is governed by the hydroxyl group present. Phenol is somewhat soluble in water because of its ability to form hydrogen. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT Aromatic Compounds PowerPoint Presentation, free download ID What Are Phenols Soluble In Water Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. However, it is more soluble in organic solvents like ether, acetone, and chloroform. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding.. What Are Phenols Soluble In Water.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in What Are Phenols Soluble In Water However, it is more soluble in organic solvents like ether, acetone, and chloroform. small alcohols are completely soluble in water; Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. If you try to dissolve more than this, you get two layers of liquid. Phenol is somewhat soluble in water because of its. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT Chapter 21, Benzene and and the Concept of Aromaticity PowerPoint What Are Phenols Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. Phenol acts as a weak acid. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Phenol is partially soluble in water. phenols are similar to alcohols but form stronger hydrogen bonds. Thus, hydrogen bonds are formed between. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free What Are Phenols Soluble In Water phenols are similar to alcohols but form stronger hydrogen bonds. Phenol acts as a weak acid. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Phenol is partially soluble in water. If you try to dissolve more than this, you get two. The hydroxyl group can release a proton (h +). What Are Phenols Soluble In Water.
From www.numerade.com
Both phenol and cyclohexanol are only slightly soluble in water What Are Phenols Soluble In Water small alcohols are completely soluble in water; Phenol acts as a weak acid. the solubility of phenol in water is governed by the hydroxyl group present. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. Mixing the two in any proportion generates a single solution. Phenol is partially soluble in water. Phenol is. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols What Are Phenols Soluble In Water If you try to dissolve more than this, you get two layers of liquid. However, it is more soluble in organic solvents like ether, acetone, and chloroform. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. small alcohols are completely soluble in water; If you try to dissolve more than this,. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT PHENOL PowerPoint Presentation, free download ID1818685 What Are Phenols Soluble In Water However, it is more soluble in organic solvents like ether, acetone, and chloroform. Phenol acts as a weak acid. Mixing the two in any proportion generates a single solution. If you try to dissolve more than this, you get two. small alcohols are completely soluble in water; the solubility of phenol in water is governed by the hydroxyl. What Are Phenols Soluble In Water.
From mutualsolubilitycurve20163b.blogspot.com
Lab Report for Experiment 3b MUTUAL SOLUBILITY CURVE FOR PHENOL AND WATER What Are Phenols Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. Phenol acts as a weak acid. If you try to dissolve more than. What Are Phenols Soluble In Water.
From www.researchgate.net
Examples of phenolic compounds. Download Scientific Diagram What Are Phenols Soluble In Water the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more than this, you get two layers of liquid. However, it is more soluble in organic solvents like ether, acetone, and chloroform. Phenol is partially soluble in water. small alcohols are completely soluble in water; phenols are similar to. What Are Phenols Soluble In Water.
From dxoiubkxb.blob.core.windows.net
What Is Phenols In Drinking Water at Judy Thompson blog What Are Phenols Soluble In Water Mixing the two in any proportion generates a single solution. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the water. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. If you try to dissolve more than this, you get two. However, it. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT Phase Equilibrium PowerPoint Presentation, free download ID5469493 What Are Phenols Soluble In Water Phenol acts as a weak acid. Phenol is partially soluble in water. Mixing the two in any proportion generates a single solution. If you try to dissolve more than this, you get two. Thus, hydrogen bonds are formed between water and phenol molecules which make phenol soluble in water. phenols are similar to alcohols but form stronger hydrogen bonds.. What Are Phenols Soluble In Water.
From www.bartleby.com
Answered 4.) Phenol Why slightly soluble in… bartleby What Are Phenols Soluble In Water phenols are similar to alcohols but form stronger hydrogen bonds. small alcohols are completely soluble in water; the solubility of phenol in water is governed by the hydroxyl group present. If you try to dissolve more than this, you get two. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with the. What Are Phenols Soluble In Water.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID2133789 What Are Phenols Soluble In Water If you try to dissolve more than this, you get two layers of liquid. Mixing the two in any proportion generates a single solution. If you try to dissolve more than this, you get two. Phenol acts as a weak acid. The hydroxyl group in phenol is involved in the formation of intermolecular hydrogen bonding. Phenol is partially soluble in. What Are Phenols Soluble In Water.
From slideplayer.com
Phenols. ppt download What Are Phenols Soluble In Water If you try to dissolve more than this, you get two. Phenol acts as a weak acid. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. Thus, they are more soluble in water than are alcohols. Phenol is somewhat soluble in water because of its ability to form hydrogen. What Are Phenols Soluble In Water.
From dxoiubkxb.blob.core.windows.net
What Is Phenols In Drinking Water at Judy Thompson blog What Are Phenols Soluble In Water phenols are similar to alcohols but form stronger hydrogen bonds. If you try to dissolve more than this, you get two. However, it is more soluble in organic solvents like ether, acetone, and chloroform. Mixing the two in any proportion generates a single solution. Phenol is somewhat soluble in water because of its ability to form hydrogen bonds with. What Are Phenols Soluble In Water.
From www.numerade.com
SOLVED Classification Physical Properties of Alcohols and Phenols What Are Phenols Soluble In Water Is phenol an acid or base. The hydroxyl group can release a proton (h +) from the oxygen atom, making phenol slightly acidic in aqueous solutions. Phenol acts as a weak acid. the solubility of phenol in water is governed by the hydroxyl group present. Thus, they are more soluble in water than are alcohols. small alcohols are. What Are Phenols Soluble In Water.
From www.numerade.com
SOLVEDAnswer true or false. (a) Phenols and alcohols have in common What Are Phenols Soluble In Water Mixing the two in any proportion generates a single solution. phenols are similar to alcohols but form stronger hydrogen bonds. Is phenol an acid or base. Thus, they are more soluble in water than are alcohols. Phenol acts as a weak acid. small alcohols are completely soluble in water; Phenol is partially soluble in water. However, it is. What Are Phenols Soluble In Water.
From dxoiubkxb.blob.core.windows.net
What Is Phenols In Drinking Water at Judy Thompson blog What Are Phenols Soluble In Water Phenol is partially soluble in water. Phenol acts as a weak acid. small alcohols are completely soluble in water; the solubility of phenol in water is governed by the hydroxyl group present. Is phenol an acid or base. Mixing the two in any proportion generates a single solution. The hydroxyl group can release a proton (h +) from. What Are Phenols Soluble In Water.