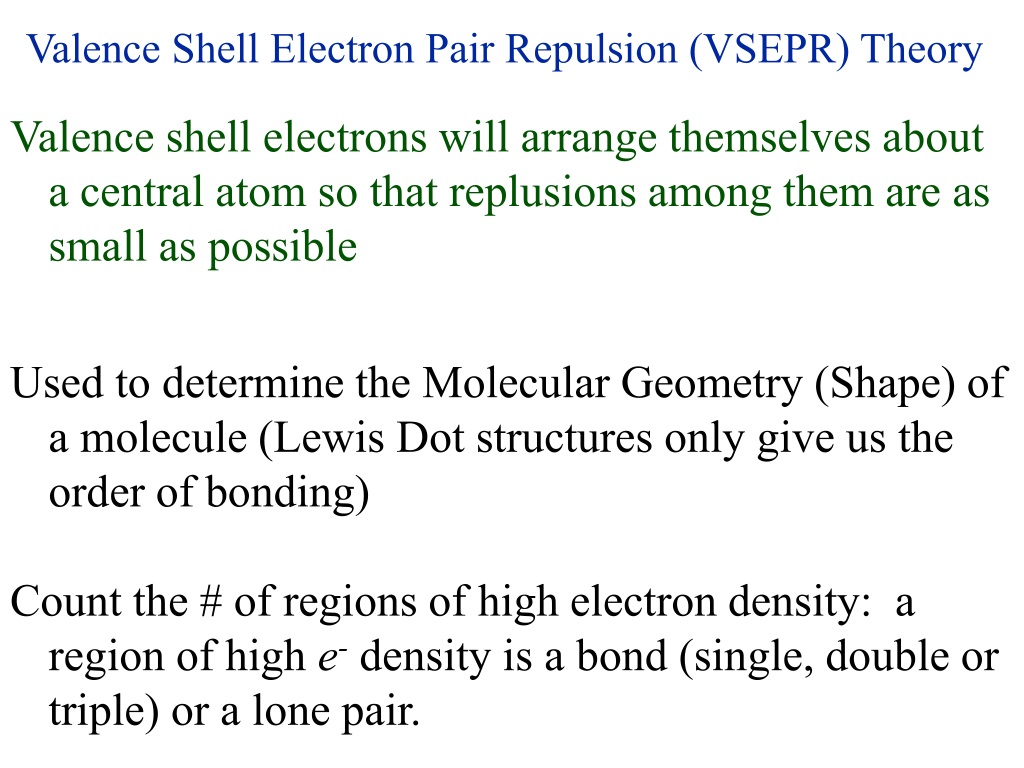
What Is Valence Shell Electron Pair . According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. The electron pairs will rearrange themselves to minimize the repulsion. It is based on the core concept that electrons repel one.
from www.slideserve.com
It is based on the core concept that electrons repel one. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. The electron pairs will rearrange themselves to minimize the repulsion. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules.
PPT Valence Shell Electron Pair Repulsion (VSEPR) Theory PowerPoint
What Is Valence Shell Electron Pair valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. The electron pairs will rearrange themselves to minimize the repulsion. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. It is based on the core concept that electrons repel one.
From www.slideserve.com
PPT Valence Shell Electron Pair Repulsion (VSEPR) theory PowerPoint What Is Valence Shell Electron Pair valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. The. What Is Valence Shell Electron Pair.
From chyscience.blogspot.com
Chemical Science Valence Shell Electron Pair Repulsion Theory What Is Valence Shell Electron Pair valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. The electron pairs will rearrange themselves to minimize the repulsion. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron pair repulsion model is often abbreviated as vsepr. What Is Valence Shell Electron Pair.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. According to this theory, the molecular shape depends on the repulsion. What Is Valence Shell Electron Pair.
From byjus.com
Different energy shells, valence shell, valence electrons What Is Valence Shell Electron Pair the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. valence shell electron pair repulsion theory is a method of. What Is Valence Shell Electron Pair.
From www.pinterest.com
Valence Shell Electron Pair Repulsion VSEPR College chemistry What Is Valence Shell Electron Pair It is based on the core concept that electrons repel one. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. The electron pairs. What Is Valence Shell Electron Pair.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Valence Shell Electron Pair The electron pairs will rearrange themselves to minimize the repulsion. It is based on the core concept that electrons repel one. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the valence shell electron repulsion (vsepr) model can predict the structure of most. What Is Valence Shell Electron Pair.
From www.sliderbase.com
Valence Shell Electron Pair Repulsion Presentation Chemistry What Is Valence Shell Electron Pair According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper). What Is Valence Shell Electron Pair.
From www.brainkart.com
Valence Shell Electron Pair Repulsion (VSEPR) theory Chemical bonding What Is Valence Shell Electron Pair It is based on the core concept that electrons repel one. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. The electron pairs will rearrange themselves to minimize the. What Is Valence Shell Electron Pair.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. valence shell electron pair repulsion theory is a method of predicting the geometry. What Is Valence Shell Electron Pair.
From biologydictionary.net
Covalent Bond Biology Dictionary What Is Valence Shell Electron Pair the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper). What Is Valence Shell Electron Pair.
From mungfali.com
Valence Electron Shell Diagram What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. The electron pairs will rearrange themselves to minimize the repulsion. According. What Is Valence Shell Electron Pair.
From www.youtube.com
Valance Shell Electron Pair Repulsion Theory YouTube What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules.. What Is Valence Shell Electron Pair.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Valence Shell Electron Pair The electron pairs will rearrange themselves to minimize the repulsion. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. the valence shell electron pair repulsion. What Is Valence Shell Electron Pair.
From studylib.net
VSEPR Valenceshell Electronpair Repulsion Theory Shows 3 What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. The electron pairs will rearrange themselves to minimize the repulsion. the vsepr theory. What Is Valence Shell Electron Pair.
From depositphotos.com
Valence Shell Electron Pair Repulsion Vsper Theory Infographic Diagram What Is Valence Shell Electron Pair valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. The electron pairs will rearrange themselves to minimize the repulsion. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron repulsion (vsepr) model can predict. What Is Valence Shell Electron Pair.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram What Is Valence Shell Electron Pair According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper). What Is Valence Shell Electron Pair.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is Valence Shell Electron Pair It is based on the core concept that electrons repel one. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. the vsepr theory assumes that each atom. What Is Valence Shell Electron Pair.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Valence Shell Electron Pair the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. The electron pairs will rearrange themselves to minimize the repulsion. According to this theory, the molecular. What Is Valence Shell Electron Pair.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is Valence Shell Electron Pair the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. The electron pairs will rearrange themselves to minimize the repulsion. It is based on the core concept that electrons repel one. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central. What Is Valence Shell Electron Pair.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. It is based on the core concept that electrons repel one. the vsepr theory assumes that each atom. What Is Valence Shell Electron Pair.
From chemistry-desk.blogspot.com
Valence Shell Electron Pair Repulsion (VSEPR) Theory │Chemistry Desk What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. the vsepr theory assumes that each atom in a molecule will achieve a geometry that. What Is Valence Shell Electron Pair.
From www.slideserve.com
PPT I. VSEPR = Valence Shell ElectronPair Repulsion Electron pairs What Is Valence Shell Electron Pair the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to. What Is Valence Shell Electron Pair.
From www.slideserve.com
PPT Valence shell electron pair repulsion (VSEPR) model PowerPoint What Is Valence Shell Electron Pair According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. It is based on the core concept that electrons repel one. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the valence. What Is Valence Shell Electron Pair.
From www.youtube.com
Valence Shell Electron Pair Repulsion Theory VSEPR Theory with What Is Valence Shell Electron Pair The electron pairs will rearrange themselves to minimize the repulsion. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron repulsion (vsepr) model can predict the structure of. What Is Valence Shell Electron Pair.
From www.brainkart.com
Valence Shell Electron Pair Repulsion (VSEPR) theory Chemical bonding What Is Valence Shell Electron Pair the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. The electron pairs will rearrange themselves to minimize the repulsion. the valence shell electron repulsion (vsepr) model can predict. What Is Valence Shell Electron Pair.
From www.slideserve.com
PPT Valence Shell Electron Pair Repulsion (VSEPR) Theory PowerPoint What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron repulsion (vsepr) model can predict the structure of most. What Is Valence Shell Electron Pair.
From www.focuskimia.com
General Chemistry Review Valence Electrons of The First Row Elements What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. The electron pairs will rearrange themselves to minimize the repulsion. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. the vsepr theory assumes that each atom in. What Is Valence Shell Electron Pair.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Is Valence Shell Electron Pair According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. The electron pairs will rearrange themselves to minimize the repulsion. valence shell electron. What Is Valence Shell Electron Pair.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is Valence Shell Electron Pair According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the vsepr theory assumes that each atom in a molecule will achieve a. What Is Valence Shell Electron Pair.
From www.slideserve.com
PPT Chapter 9 Molecular Geometry and Bonding Theories PowerPoint What Is Valence Shell Electron Pair the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. It is based on the core concept that electrons repel one.. What Is Valence Shell Electron Pair.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary What Is Valence Shell Electron Pair the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. The electron pairs will rearrange themselves to minimize the repulsion. According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. It is based on the core concept that electrons repel. What Is Valence Shell Electron Pair.
From www.youtube.com
Molecular Geometry Part 2Valence Shell Electron Pair Repulsion theory What Is Valence Shell Electron Pair the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron repulsion (vsepr) model can predict the. What Is Valence Shell Electron Pair.
From surfguppy.com
Valence Electrons Definition, Obits and Energy Level What Is Valence Shell Electron Pair According to this theory, the molecular shape depends on the repulsion between the valence shell electron pairs of the central atom. The electron pairs will rearrange themselves to minimize the repulsion. valence shell electron pair repulsion theory is a method of predicting the geometry of molecules. the valence shell electron repulsion (vsepr) model can predict the structure of. What Is Valence Shell Electron Pair.
From classnotes.org.in
Valence Shell Electron Pair Repulsion Theory Chemical Bonding and What Is Valence Shell Electron Pair It is based on the core concept that electrons repel one. the valence shell electron repulsion (vsepr) model can predict the structure of most molecules and polyatomic ions in. The electron pairs will rearrange themselves to minimize the repulsion. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between. What Is Valence Shell Electron Pair.
From www.youtube.com
Valence Electrons and the Periodic Table YouTube What Is Valence Shell Electron Pair The electron pairs will rearrange themselves to minimize the repulsion. the vsepr theory assumes that each atom in a molecule will achieve a geometry that minimizes the repulsion between electrons in the valence. the valence shell electron pair repulsion model is often abbreviated as vsepr (pronounced vesper) and is a model to predict the geometry of molecules. . What Is Valence Shell Electron Pair.