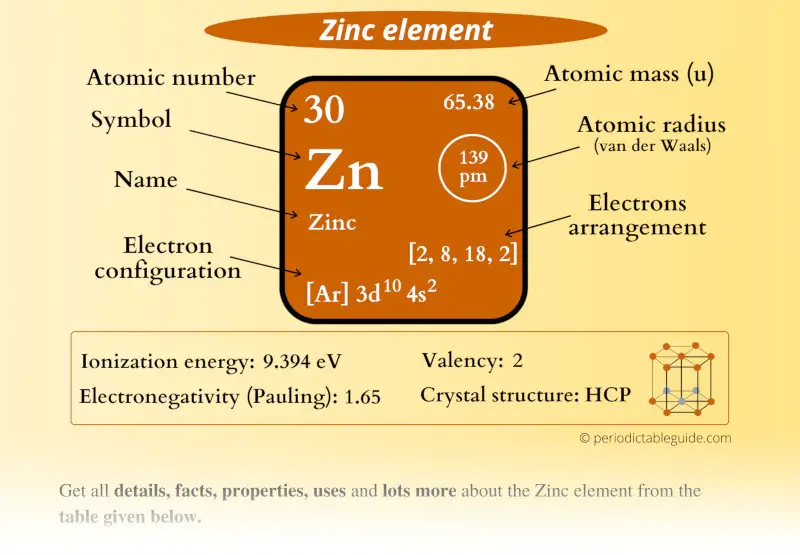
Electrons Does Zinc Have . It is used for galvanising, alloys, oxide, sulfide and other. It has an atomic weight of 65.38 and a mass. It is a moderately reactive metal and strong reducing agent. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. This means that neutral zinc atom has a total of 30 electrons surrounding its.
from elchoroukhost.net
Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. It is used for galvanising, alloys, oxide, sulfide and other. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It has an atomic weight of 65.38 and a mass. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. This means that neutral zinc atom has a total of 30 electrons surrounding its. It is a moderately reactive metal and strong reducing agent. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus.
Periodic Table Zinc Protons Neutrons Electrons Elcho Table
Electrons Does Zinc Have 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. It is used for galvanising, alloys, oxide, sulfide and other. It is a moderately reactive metal and strong reducing agent. It has an atomic weight of 65.38 and a mass. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. This means that neutral zinc atom has a total of 30 electrons surrounding its.
From mungfali.com
Zinc Orbital Diagram Electrons Does Zinc Have Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. This means that neutral zinc atom has a total of 30 electrons surrounding its. Zinc is a chemical element with. Electrons Does Zinc Have.
From periodictable.me
Zinc Valence Electron Dot Diagram Archives Dynamic Periodic Table of Electrons Does Zinc Have 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. It is used for galvanising, alloys, oxide, sulfide and other. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Total number of protons in the nucleus is called. Electrons Does Zinc Have.
From www.youtube.com
How many valence electrons does each of the following dietary trace Electrons Does Zinc Have 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. It is used for galvanising, alloys, oxide, sulfide and other. This means that neutral zinc atom has a total of 30 electrons surrounding its. Total number of protons in the nucleus is called the atomic number of the atom. Electrons Does Zinc Have.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Electrons Does Zinc Have Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is used for galvanising, alloys, oxide, sulfide and other. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. This means that neutral zinc atom has a total. Electrons Does Zinc Have.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Electrons Does Zinc Have It is used for galvanising, alloys, oxide, sulfide and other. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Zinc is the 30th element in the periodic table and. Electrons Does Zinc Have.
From valenceelectrons.com
How many protons, neutrons and electrons does zinc have? Electrons Does Zinc Have Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. It is a moderately reactive metal and strong reducing agent. It is used for galvanising, alloys, oxide, sulfide and other. It has. Electrons Does Zinc Have.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Electrons Does Zinc Have This means that neutral zinc atom has a total of 30 electrons surrounding its. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Zinc is the 30th element in the. Electrons Does Zinc Have.
From animalia-life.club
Zinc Electron Configuration Electrons Does Zinc Have It is a moderately reactive metal and strong reducing agent. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It has an atomic weight of 65.38 and a. Electrons Does Zinc Have.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Electrons Does Zinc Have It has an atomic weight of 65.38 and a mass. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Zinc is located in period 4, group. Electrons Does Zinc Have.
From animalia-life.club
Zinc Electron Configuration Electrons Does Zinc Have It has an atomic weight of 65.38 and a mass. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is used for galvanising, alloys, oxide, sulfide and other. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of. Electrons Does Zinc Have.
From golace.blogspot.com
zinc orbital diagram Golace Electrons Does Zinc Have This means that neutral zinc atom has a total of 30 electrons surrounding its. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. 93 rows you may assume. Electrons Does Zinc Have.
From www.youtube.com
How many valence electrons does zinc have? YouTube Electrons Does Zinc Have This means that neutral zinc atom has a total of 30 electrons surrounding its. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It is a moderately. Electrons Does Zinc Have.
From www.adda247.com
What is the valency of zinc? Electrons Does Zinc Have It is used for galvanising, alloys, oxide, sulfide and other. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Zinc is a chemical element with atomic number 30. Electrons Does Zinc Have.
From valenceelectrons.com
How many protons, neutrons and electrons does zinc have? Electrons Does Zinc Have This means that neutral zinc atom has a total of 30 electrons surrounding its. It is used for galvanising, alloys, oxide, sulfide and other. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. 93 rows you may assume the valences of the chemical elements—the number of electrons with which. Electrons Does Zinc Have.
From www.sciencephoto.com
Zinc, atomic structure Stock Image C013/1553 Science Photo Library Electrons Does Zinc Have It has an atomic weight of 65.38 and a mass. It is a moderately reactive metal and strong reducing agent. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol. Electrons Does Zinc Have.
From www.alamy.com
Symbol and electron diagram for Zinc illustration Stock Vector Image Electrons Does Zinc Have It has an atomic weight of 65.38 and a mass. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. This means that neutral zinc atom has a total of 30. Electrons Does Zinc Have.
From www.slideserve.com
PPT PS4 The Atom & the Periodic Table PowerPoint Presentation ID Electrons Does Zinc Have 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. This means that neutral zinc atom has a total of 30 electrons surrounding its. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It is a. Electrons Does Zinc Have.
From www.freepik.com
Free Vector A zinc atom diagram Electrons Does Zinc Have Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. It is used for galvanising, alloys, oxide, sulfide and other. This means that neutral zinc atom has a total of 30 electrons. Electrons Does Zinc Have.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Electrons Does Zinc Have It has an atomic weight of 65.38 and a mass. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. It is a moderately reactive metal and strong reducing agent. This means that neutral zinc atom has a total of 30 electrons surrounding its. 93 rows you may assume the. Electrons Does Zinc Have.
From thefitnessmanual.com
How Many Electrons Does Zinc Have TheFitnessManual Electrons Does Zinc Have Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. This means that neutral zinc atom has a total of 30 electrons surrounding its. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. It is used for galvanising,. Electrons Does Zinc Have.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Electrons Does Zinc Have It is a moderately reactive metal and strong reducing agent. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Zinc is the 30th element in the. Electrons Does Zinc Have.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Electrons Does Zinc Have It has an atomic weight of 65.38 and a mass. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It is a moderately reactive metal and strong reducing agent.. Electrons Does Zinc Have.
From animalia-life.club
Zinc Electron Configuration Electrons Does Zinc Have It is a moderately reactive metal and strong reducing agent. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It has an atomic weight of 65.38 and a mass. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Electrons Does Zinc Have.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Electrons Does Zinc Have Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It is a moderately reactive metal and strong reducing agent. 93 rows you may assume the valences of the chemical. Electrons Does Zinc Have.
From animalia-life.club
Zinc Electron Configuration Electrons Does Zinc Have It has an atomic weight of 65.38 and a mass. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. This means that neutral zinc atom has a total of. Electrons Does Zinc Have.
From valenceelectrons.com
How many protons, neutrons and electrons does zinc have? Electrons Does Zinc Have 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. It has an atomic weight of 65.38 and a mass. It is used for galvanising, alloys, oxide, sulfide and other. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of. Electrons Does Zinc Have.
From en.academic.ru
Zinc Electrons Does Zinc Have Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. It is used for galvanising, alloys, oxide, sulfide and other. 93 rows you may assume the valences of the. Electrons Does Zinc Have.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Electrons Does Zinc Have This means that neutral zinc atom has a total of 30 electrons surrounding its. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. It has an atomic weight of. Electrons Does Zinc Have.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Electrons Does Zinc Have Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. This means that neutral zinc atom has a total of 30 electrons surrounding its. It is used for galvanising, alloys, oxide,. Electrons Does Zinc Have.
From elchoroukhost.net
Zinc Periodic Table Protons Elcho Table Electrons Does Zinc Have It is a moderately reactive metal and strong reducing agent. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. This means that neutral zinc atom has a total of 30. Electrons Does Zinc Have.
From www.dreamstime.com
Zinc stock illustration. Illustration of render, chemistry 139650988 Electrons Does Zinc Have It is used for galvanising, alloys, oxide, sulfide and other. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. It is a moderately reactive metal and strong reducing agent. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. Zinc has. Electrons Does Zinc Have.
From valenceelectrons.com
How many valence electrons does zinc(Zn) have? Electrons Does Zinc Have It has an atomic weight of 65.38 and a mass. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. Zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. 93 rows you may assume the valences of the chemical elements—the number. Electrons Does Zinc Have.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Zinc (Zn Electrons Does Zinc Have 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. It is used for galvanising, alloys, oxide, sulfide and other. Zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. This means that neutral zinc atom has. Electrons Does Zinc Have.
From ar.inspiredpencil.com
Zinc Electron Configuration Electrons Does Zinc Have Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Zinc is a chemical element with atomic number 30 which means there are 30 protons in its nucleus. It is a moderately reactive metal and strong reducing agent. Zinc is the 30th element in the periodic table and has a. Electrons Does Zinc Have.
From periodictable.me
Zinc Electron Configuration (Zn) with Orbital Diagram Electrons Does Zinc Have This means that neutral zinc atom has a total of 30 electrons surrounding its. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol z. Zinc is located in period 4, group 12 of the periodic table and has an atomic number equal to 30. Zinc is the 30th element. Electrons Does Zinc Have.