How Does Surface Tension Affect Boiling Point . Surface tension is the energy required to increase the surface area of a liquid by a given amount. Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The strength of intermolecular forces (and therefore impact. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The intermolecular forces increase with increasing polarization of bonds. How intermolecular forces influence physical properties. This effect results from the difference between the potential energy.
from diagramfricanofc.z21.web.core.windows.net
Surface tension is the energy required to increase the surface area of a liquid by a given amount. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The intermolecular forces increase with increasing polarization of bonds. The strength of intermolecular forces (and therefore impact. Since these intermolecular forces vary. How intermolecular forces influence physical properties. This effect results from the difference between the potential energy. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces.
Initial Boiling Point And Final Boiling Point
How Does Surface Tension Affect Boiling Point This effect results from the difference between the potential energy. How intermolecular forces influence physical properties. The strength of intermolecular forces (and therefore impact. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This effect results from the difference between the potential energy. The intermolecular forces increase with increasing polarization of bonds. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. Since these intermolecular forces vary.
From www.youtube.com
Intermolecular Forces Trends Melting & Boiling Point, Viscosity How Does Surface Tension Affect Boiling Point The intermolecular forces increase with increasing polarization of bonds. The strength of intermolecular forces (and therefore impact. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary.. How Does Surface Tension Affect Boiling Point.
From byjus.com
How does external pressure affect the boiling point How Does Surface Tension Affect Boiling Point The strength of intermolecular forces (and therefore impact. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. Surface tension is. How Does Surface Tension Affect Boiling Point.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point How Does Surface Tension Affect Boiling Point The intermolecular forces increase with increasing polarization of bonds. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Since these intermolecular forces vary. The strength of intermolecular forces (and therefore impact. Surface tension is the energy required to increase the surface area of a liquid by a given amount. How intermolecular. How Does Surface Tension Affect Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps How Does Surface Tension Affect Boiling Point Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This effect results from the difference between the potential energy. The strength of intermolecular forces (and therefore impact. How intermolecular forces influence physical properties. Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area. How Does Surface Tension Affect Boiling Point.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID How Does Surface Tension Affect Boiling Point This effect results from the difference between the potential energy. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. How intermolecular forces influence physical properties. Surface tension is the energy required. How Does Surface Tension Affect Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps How Does Surface Tension Affect Boiling Point Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. This effect results from the difference between the potential energy. How intermolecular forces influence physical properties. The strength of intermolecular forces (and therefore impact. The intermolecular forces increase with increasing polarization. How Does Surface Tension Affect Boiling Point.
From sites.google.com
Raven Research Effects of Temperature and Salt on Surface Tension How Does Surface Tension Affect Boiling Point This effect results from the difference between the potential energy. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. How intermolecular forces influence physical properties. Cohesive forces between molecules cause the. How Does Surface Tension Affect Boiling Point.
From byjus.com
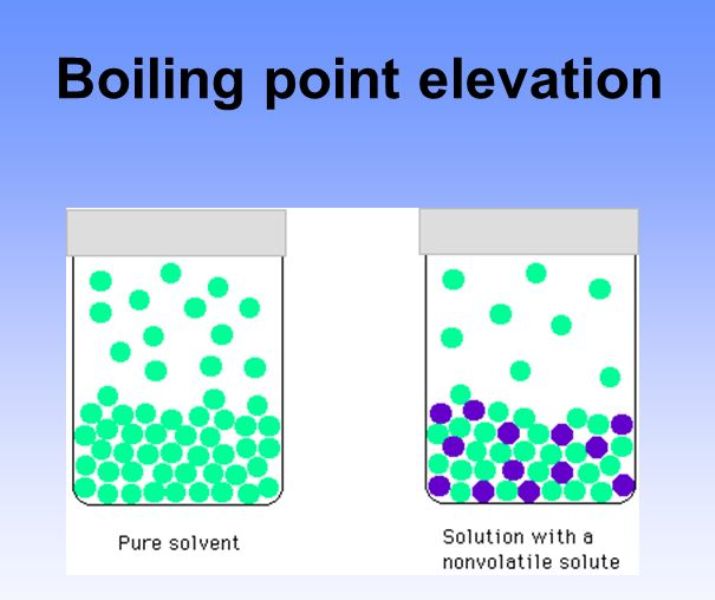
How does Solute affect Boiling Point? How Does Surface Tension Affect Boiling Point Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The strength of intermolecular forces (and therefore impact. Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Intermolecular forces have varying strengths, and the strength of. How Does Surface Tension Affect Boiling Point.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples How Does Surface Tension Affect Boiling Point The intermolecular forces increase with increasing polarization of bonds. The strength of intermolecular forces (and therefore impact. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Cohesive forces between molecules cause. How Does Surface Tension Affect Boiling Point.
From learningschooltrkesp5v.z22.web.core.windows.net
Viscosity Vs Surface Tension How Does Surface Tension Affect Boiling Point Surface tension is the energy required to increase the surface area of a liquid by a given amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This effect results from the difference between the potential energy. The strength of intermolecular forces (and therefore impact. Surface tension is the energy, or. How Does Surface Tension Affect Boiling Point.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit How Does Surface Tension Affect Boiling Point The intermolecular forces increase with increasing polarization of bonds. How intermolecular forces influence physical properties. Surface tension is the energy required to increase the surface area of a liquid by a given amount. This effect results from the difference between the potential energy. Surface tension is the energy, or work, required to increase the surface area of a liquid due. How Does Surface Tension Affect Boiling Point.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it How Does Surface Tension Affect Boiling Point Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. The intermolecular forces increase with increasing polarization of bonds. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Since these intermolecular forces. How Does Surface Tension Affect Boiling Point.
From www.youtube.com
Surface Tension of Water Explained YouTube How Does Surface Tension Affect Boiling Point Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity,. How Does Surface Tension Affect Boiling Point.
From chemistnotes.com
Elevation of Boiling Point Definition/Equation/Molal Boiling Point How Does Surface Tension Affect Boiling Point Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. The strength of intermolecular forces (and therefore impact. How intermolecular forces influence physical properties. Surface tension is the energy required to increase the surface area of a liquid by a given. How Does Surface Tension Affect Boiling Point.
From chem.libretexts.org
11.4 Intermolecular Forces in Action Surface Tension, Viscosity, and How Does Surface Tension Affect Boiling Point How intermolecular forces influence physical properties. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Since these intermolecular forces vary. Cohesive forces. How Does Surface Tension Affect Boiling Point.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance How Does Surface Tension Affect Boiling Point Since these intermolecular forces vary. The intermolecular forces increase with increasing polarization of bonds. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. How intermolecular forces influence physical properties. The strength of intermolecular forces (and therefore impact. Surface tension is. How Does Surface Tension Affect Boiling Point.
From www.youtube.com
Explaining Boiling Point (Simple Covalent, Intermolecular Forces How Does Surface Tension Affect Boiling Point This effect results from the difference between the potential energy. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Intermolecular forces have varying strengths, and the strength of the intermolecular forces. How Does Surface Tension Affect Boiling Point.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 How Does Surface Tension Affect Boiling Point The strength of intermolecular forces (and therefore impact. Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area of a liquid by a given amount. The intermolecular forces increase with increasing polarization of bonds. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties,. How Does Surface Tension Affect Boiling Point.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting How Does Surface Tension Affect Boiling Point Surface tension is the energy required to increase the surface area of a liquid by a given amount. How intermolecular forces influence physical properties. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s. How Does Surface Tension Affect Boiling Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps How Does Surface Tension Affect Boiling Point How intermolecular forces influence physical properties. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to. How Does Surface Tension Affect Boiling Point.
From www.youtube.com
Boiling point and external pressure YouTube How Does Surface Tension Affect Boiling Point Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This effect results from the difference between the potential energy. How intermolecular forces influence physical properties. Since these intermolecular forces vary. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. How Does Surface Tension Affect Boiling Point.
From www.numerade.com
SOLVED (intermolecular forces) affect the properties of compounds How Does Surface Tension Affect Boiling Point Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. This effect results from the difference between the potential energy. The strength of. How Does Surface Tension Affect Boiling Point.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox How Does Surface Tension Affect Boiling Point Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Since these intermolecular forces vary. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical. How Does Surface Tension Affect Boiling Point.
From www.youtube.com
The surface tension of liquid at its boiling point YouTube How Does Surface Tension Affect Boiling Point Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. The strength of intermolecular forces (and therefore impact. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. This effect results from the. How Does Surface Tension Affect Boiling Point.
From wagine.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps How Does Surface Tension Affect Boiling Point How intermolecular forces influence physical properties. The strength of intermolecular forces (and therefore impact. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure,. How Does Surface Tension Affect Boiling Point.
From www.slideserve.com
PPT Chapter 6 Intermolecular Forces of Attractions Between Particles How Does Surface Tension Affect Boiling Point Since these intermolecular forces vary. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The strength of intermolecular forces (and therefore impact. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor. How Does Surface Tension Affect Boiling Point.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs How Does Surface Tension Affect Boiling Point Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Surface tension is the energy required to increase the surface area. How Does Surface Tension Affect Boiling Point.
From byjus.com
Explain the surface tension phenomenon with examples. How Does Surface Tension Affect Boiling Point Surface tension is the energy required to increase the surface area of a liquid by a given amount. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. How intermolecular forces influence physical properties. The intermolecular forces increase with increasing polarization of bonds. Since these intermolecular forces vary. This effect results from. How Does Surface Tension Affect Boiling Point.
From www.merchantnavydecoded.com
What is Surface tension? Why does surface tension occur? Merchant How Does Surface Tension Affect Boiling Point The strength of intermolecular forces (and therefore impact. How intermolecular forces influence physical properties. Surface tension is the energy required to increase the surface area of a liquid by a given amount. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This effect results from the difference between the. How Does Surface Tension Affect Boiling Point.
From www.elephango.com
Tension in the Water Educational Resources K12 Learning, Physical How Does Surface Tension Affect Boiling Point Since these intermolecular forces vary. The strength of intermolecular forces (and therefore impact. The intermolecular forces increase with increasing polarization of bonds. This effect results from the difference between the potential energy. How intermolecular forces influence physical properties. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is the. How Does Surface Tension Affect Boiling Point.
From www.slideserve.com
PPT Polarity and Intermolecular Forces PowerPoint Presentation, free How Does Surface Tension Affect Boiling Point Since these intermolecular forces vary. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. How intermolecular forces influence physical properties. This effect results from the difference between the potential energy. The strength of intermolecular forces (and therefore impact. The intermolecular. How Does Surface Tension Affect Boiling Point.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID6036532 How Does Surface Tension Affect Boiling Point Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. This effect results from the difference between the potential energy. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Surface tension is. How Does Surface Tension Affect Boiling Point.
From www.slideserve.com
PPT Intermolecular Forces and Liquids and Solids PowerPoint How Does Surface Tension Affect Boiling Point The intermolecular forces increase with increasing polarization of bonds. Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. Cohesive forces. How Does Surface Tension Affect Boiling Point.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) How Does Surface Tension Affect Boiling Point Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. How intermolecular forces influence physical properties. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This effect results from the difference between. How Does Surface Tension Affect Boiling Point.
From www.biolinscientific.com
Surface tension of water Why is it so high? How Does Surface Tension Affect Boiling Point The strength of intermolecular forces (and therefore impact. Intermolecular forces have varying strengths, and the strength of the intermolecular forces within a substance influences the substance’s physical properties, including boiling/melting points, viscosity, surface tension, vapor pressure, etc. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. How intermolecular forces influence physical. How Does Surface Tension Affect Boiling Point.