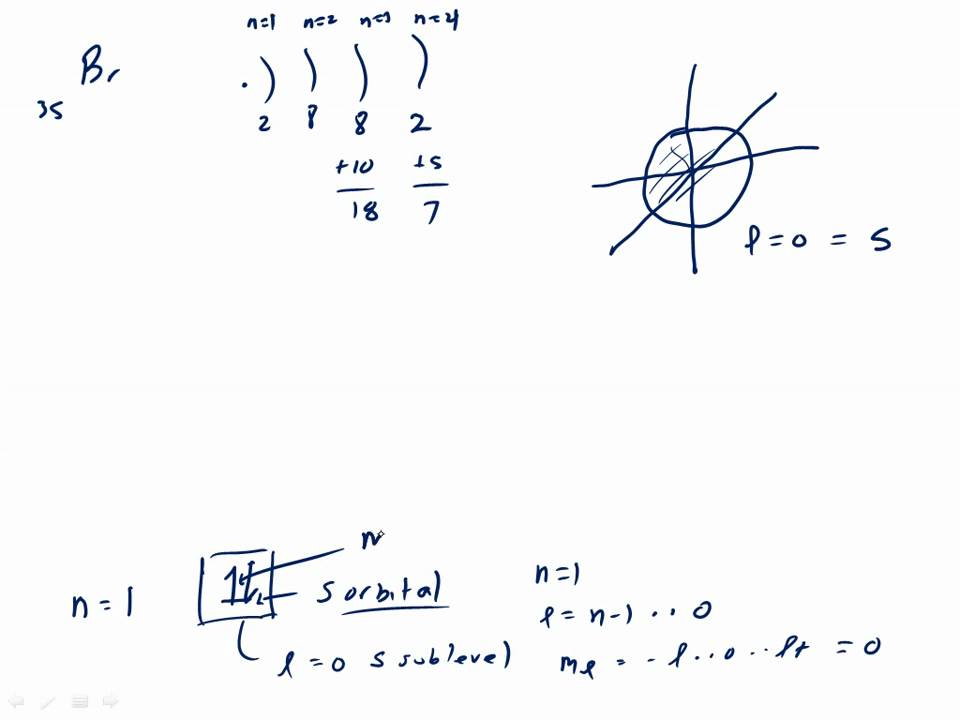
Bromine Orbital Filling Diagram . For example, bromine takes one electron and becomes isoelectronic to t kr: That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Now it is possible to find the orbital notation of bromine very easily through electron configuration. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. To write the orbital diagram for the bromine atom (br) first we need to write the electron. The atomic number of bromine is 35, which means it has 35 electrons. The order of the filling is from bottom to top,.
from schematron.org
That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. The order of the filling is from bottom to top,. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. Now it is possible to find the orbital notation of bromine very easily through electron configuration. For example, bromine takes one electron and becomes isoelectronic to t kr: The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. The atomic number of bromine is 35, which means it has 35 electrons. To write the orbital diagram for the bromine atom (br) first we need to write the electron.
Show The Orbitalfilling Diagram For Br (bromine) Wiring Diagram Pictures
Bromine Orbital Filling Diagram The atomic number of bromine is 35, which means it has 35 electrons. To write the orbital diagram for the bromine atom (br) first we need to write the electron. Now it is possible to find the orbital notation of bromine very easily through electron configuration. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. The order of the filling is from bottom to top,. For example, bromine takes one electron and becomes isoelectronic to t kr: The atomic number of bromine is 35, which means it has 35 electrons. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic.
From wiringlistvoltairean.z14.web.core.windows.net
How To Write Electron Configuration Diagrams Bromine Orbital Filling Diagram The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. The atomic number of bromine is 35, which means it has 35 electrons. To write the orbital diagram for the bromine atom. Bromine Orbital Filling Diagram.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Bromine Orbital Filling Diagram That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Now it is possible to find the orbital notation of bromine very easily through electron configuration. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic.. Bromine Orbital Filling Diagram.
From diagramweb.net
Orbital Filling Diagram For Bromine Bromine Orbital Filling Diagram To write the orbital diagram for the bromine atom (br) first we need to write the electron. That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. Now. Bromine Orbital Filling Diagram.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Orbital Filling Diagram The atomic number of bromine is 35, which means it has 35 electrons. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. The order of the filling is from bottom to top,. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6). Bromine Orbital Filling Diagram.
From wiringall.com
Bromine Orbital Diagram Bromine Orbital Filling Diagram The atomic number of bromine is 35, which means it has 35 electrons. The order of the filling is from bottom to top,. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. Now it is possible to find the orbital notation of bromine very easily through electron configuration. That. Bromine Orbital Filling Diagram.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Orbital Filling Diagram In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. Now it is possible to find the orbital notation of bromine very easily through electron configuration.. Bromine Orbital Filling Diagram.
From schematiclistpact101.z22.web.core.windows.net
Orbital Diagram Configuration Bromine Orbital Filling Diagram That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Now it is possible to find the orbital notation of bromine very easily through electron configuration. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. For example,. Bromine Orbital Filling Diagram.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle Bromine Orbital Filling Diagram The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Now it is possible to find the orbital notation of bromine very easily through electron configuration. To write. Bromine Orbital Filling Diagram.
From schematron.org
Show The Orbitalfilling Diagram For Br (bromine) Wiring Diagram Pictures Bromine Orbital Filling Diagram The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. Now it is possible to find the orbital notation of bromine very easily through electron configuration. To write the orbital diagram for the bromine atom (br) first we need to write the electron. In the case of br2, the molecular orbital diagram. Bromine Orbital Filling Diagram.
From roomiladeejay.blogspot.com
5+ orbital diagram for neon RoomilaDeejay Bromine Orbital Filling Diagram For example, bromine takes one electron and becomes isoelectronic to t kr: The order of the filling is from bottom to top,. The atomic number of bromine is 35, which means it has 35 electrons. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. To write the orbital diagram for the. Bromine Orbital Filling Diagram.
From schematron.org
Bromine Orbital Diagram Wiring Diagram Pictures Bromine Orbital Filling Diagram Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. To write the orbital diagram for the bromine atom (br) first we need to write the electron. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. That is, the orbital notation. Bromine Orbital Filling Diagram.
From quizlet.com
How to draw the orbital diagram of bromine? Quizlet Bromine Orbital Filling Diagram In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. The order of the filling is from bottom to top,. For example, bromine takes one electron and becomes isoelectronic to t kr: To write the orbital diagram for the bromine atom (br) first we. Bromine Orbital Filling Diagram.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Bromine (Br) YouTube Bromine Orbital Filling Diagram The order of the filling is from bottom to top,. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. Now it is possible to find the orbital notation of bromine very easily through electron configuration. Br([ar] 4s 2 3d 10 4p 5) +. Bromine Orbital Filling Diagram.
From www.bartleby.com
Answered Show the orbitalfilling diagram for Br… bartleby Bromine Orbital Filling Diagram For example, bromine takes one electron and becomes isoelectronic to t kr: That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Now it is possible to find the orbital notation of bromine very easily through electron configuration. To write the orbital diagram for the bromine. Bromine Orbital Filling Diagram.
From schematiclistsalem123.z22.web.core.windows.net
Orbital Diagram Vs Electron Configuration Bromine Orbital Filling Diagram To write the orbital diagram for the bromine atom (br) first we need to write the electron. For example, bromine takes one electron and becomes isoelectronic to t kr: Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. Now it is possible to find the orbital notation of bromine. Bromine Orbital Filling Diagram.
From diagramweb.net
Orbital Filling Diagram For Bromine Bromine Orbital Filling Diagram The order of the filling is from bottom to top,. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. The atomic number of bromine is 35, which means it has 35 electrons. The orbital filling diagram for bromine can help us understand the. Bromine Orbital Filling Diagram.
From quizlet.com
Show the orbitalfilling diagram for Br (bromine). Quizlet Bromine Orbital Filling Diagram Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. The order of the filling is from bottom to top,. For example, bromine takes one electron and becomes isoelectronic to t kr: That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6. Bromine Orbital Filling Diagram.
From wiringall.com
Show The Orbital Filling Diagram For Br Bromine Bromine Orbital Filling Diagram The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. To write the orbital diagram for the bromine atom (br) first we need to write the. Bromine Orbital Filling Diagram.
From manualfixthanedom77.z22.web.core.windows.net
What Is A Orbital Diagram Bromine Orbital Filling Diagram The order of the filling is from bottom to top,. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. The atomic number of bromine is 35, which. Bromine Orbital Filling Diagram.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Bromine Orbital Filling Diagram To write the orbital diagram for the bromine atom (br) first we need to write the electron. The order of the filling is from bottom to top,. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals. Bromine Orbital Filling Diagram.
From wiringall.com
Show The Orbital Filling Diagram For Br Bromine Bromine Orbital Filling Diagram The atomic number of bromine is 35, which means it has 35 electrons. To write the orbital diagram for the bromine atom (br) first we need to write the electron. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. The order of the. Bromine Orbital Filling Diagram.
From wiringall.com
Bromine Orbital Diagram Bromine Orbital Filling Diagram The order of the filling is from bottom to top,. That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Now it is possible to find the orbital notation of bromine very easily through electron configuration. In the case of br2, the molecular orbital diagram shows. Bromine Orbital Filling Diagram.
From wiringdiagram99.blogspot.com
Orbital Filling Diagram For Bromine Wiring Diagram Database Bromine Orbital Filling Diagram That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. Now it is possible to find the orbital notation of bromine very easily through electron configuration.. Bromine Orbital Filling Diagram.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Bromine Orbital Filling Diagram That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5. To write the orbital diagram for the bromine atom (br) first we need to write the electron. The order of the filling is from bottom to top,. Br([ar] 4s 2 3d 10 4p 5) + e. Bromine Orbital Filling Diagram.
From quizlet.com
How to draw the orbital diagram of bromine? Quizlet Bromine Orbital Filling Diagram For example, bromine takes one electron and becomes isoelectronic to t kr: Now it is possible to find the orbital notation of bromine very easily through electron configuration. The atomic number of bromine is 35, which means it has 35 electrons. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms. Bromine Orbital Filling Diagram.
From diagramweb.net
Orbital Filling Diagram For Bromine Bromine Orbital Filling Diagram The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. Now it is possible to find the orbital notation of bromine very easily through electron configuration. The atomic number of bromine is 35, which means it has 35 electrons. For example, bromine takes one electron and becomes isoelectronic to t kr: In. Bromine Orbital Filling Diagram.
From manuallistcantabank.z21.web.core.windows.net
Orbital Filling Diagram Bromine Orbital Filling Diagram The order of the filling is from bottom to top,. To write the orbital diagram for the bromine atom (br) first we need to write the electron. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. Now it is possible to find the. Bromine Orbital Filling Diagram.
From guidefixternur9z.z22.web.core.windows.net
Molecular Orbital Diagram Calculator Bromine Orbital Filling Diagram The atomic number of bromine is 35, which means it has 35 electrons. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. For example, bromine. Bromine Orbital Filling Diagram.
From mungfali.com
Aufbau Diagram For Bromine Bromine Orbital Filling Diagram For example, bromine takes one electron and becomes isoelectronic to t kr: The atomic number of bromine is 35, which means it has 35 electrons. Br([ar] 4s 2 3d 10 4p 5) + e − br − ([ar] 4s 2 3d 10 4p 6) [isoelectronic. Now it is possible to find the orbital notation of bromine very easily through electron. Bromine Orbital Filling Diagram.
From ar.inspiredpencil.com
Orbital Diagram Of Bromine Bromine Orbital Filling Diagram The atomic number of bromine is 35, which means it has 35 electrons. The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. Now it is possible to find the orbital notation of bromine very easily through electron configuration. The order of the filling is from bottom to top,. In the case. Bromine Orbital Filling Diagram.
From wiringfixunripping.z21.web.core.windows.net
Valence Electrons Orbital Diagram Bromine Orbital Filling Diagram The order of the filling is from bottom to top,. The atomic number of bromine is 35, which means it has 35 electrons. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. That is, the orbital notation of bromine is 1s 2 2s. Bromine Orbital Filling Diagram.
From wizedu.com
Show the orbitalfilling diagram for Br (bromine). Order subshells by Bromine Orbital Filling Diagram The orbital filling diagram for bromine can help us understand the arrangement of electrons in its atomic structure. In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. The order of the filling is from bottom to top,. Br([ar] 4s 2 3d 10 4p. Bromine Orbital Filling Diagram.
From jumpstarterdiscount.blogspot.com
Show The Orbital Filling Diagram For Br Bromine Wiring Diagram Bromine Orbital Filling Diagram For example, bromine takes one electron and becomes isoelectronic to t kr: In the case of br2, the molecular orbital diagram shows that the two 2s orbitals of the bromine atoms combine to form a sigma bonding orbital. That is, the orbital notation of bromine is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Bromine Orbital Filling Diagram.