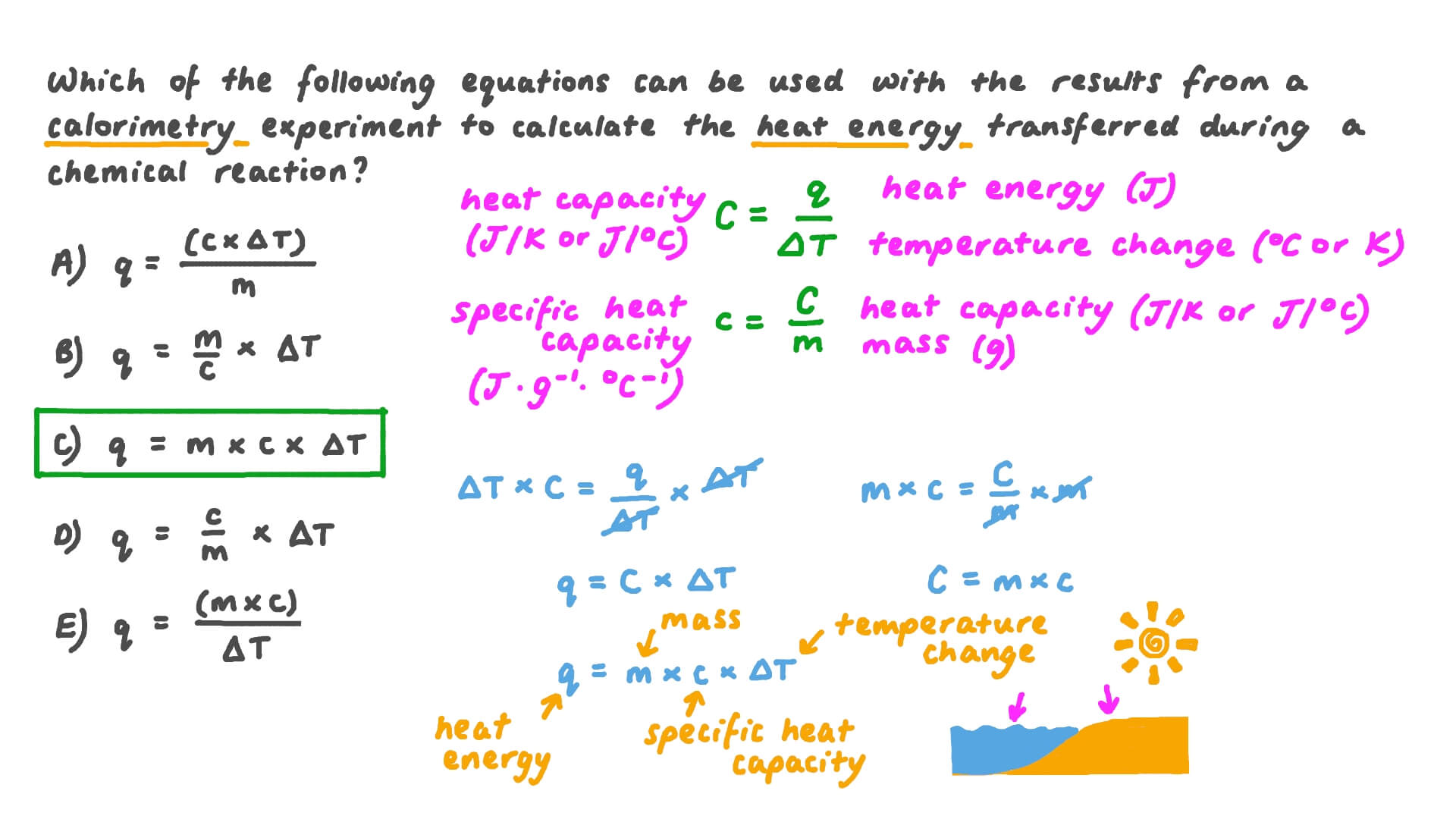Bomb Calorimetry Questions . If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: here is a sample problem for you to solve. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. A 0.500 gram sample of. this page titled 7.4: The heat measured in a bomb calorimeter for a reaction is:
from exoepufyb.blob.core.windows.net
here is a sample problem for you to solve. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. A 0.500 gram sample of. The heat measured in a bomb calorimeter for a reaction is: If the heat capacity of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: this page titled 7.4:
Calorimetry Multiple Choice Questions at Candace Cottingham blog
Bomb Calorimetry Questions 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: here is a sample problem for you to solve. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: A 0.500 gram sample of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. If the heat capacity of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. this page titled 7.4: The heat measured in a bomb calorimeter for a reaction is: If the heat capacity of.
From www.chegg.com
Solved QUESTIONS FOR BOMB CALORIMETRY EXP. NAME 1. What kind Bomb Calorimetry Questions this page titled 7.4: If the heat capacity of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. The heat measured in a bomb calorimeter for a reaction is: 4) since this is a bomb calorimeter (a constant volume apparatus), we can. Bomb Calorimetry Questions.
From www.chegg.com
Solved The Model Bomb Calorimetry Motorized stirrer Bomb Calorimetry Questions this page titled 7.4: 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6. Bomb Calorimetry Questions.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Bomb Calorimetry Questions when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. this page titled 7.4: when 1.00 g of coal is. Bomb Calorimetry Questions.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimetry Questions a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship:. Bomb Calorimetry Questions.
From www.chegg.com
Solved Experiment name Bomb Calorimetry Heat of Combustion Bomb Calorimetry Questions a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. here is a sample problem for you to solve. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: The heat measured in a bomb calorimeter for. Bomb Calorimetry Questions.
From www.chegg.com
Solved Question 1 We have a bomb calorimeter with a heat Bomb Calorimetry Questions here is a sample problem for you to solve. this page titled 7.4: If the heat capacity of. If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid. Bomb Calorimetry Questions.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Bomb Calorimetry Questions The heat measured in a bomb calorimeter for a reaction is: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. A 0.500 gram sample of. this page titled 7.4: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. here is. Bomb Calorimetry Questions.
From studydbhertz.z21.web.core.windows.net
How To Calculate Calorimetry Problems Bomb Calorimetry Questions 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: A 0.500 gram sample of. here is a sample problem for you to solve. this page titled 7.4: The heat measured in a bomb calorimeter for a reaction is: If the heat capacity of. when 1.00 g of coal is. Bomb Calorimetry Questions.
From www.numerade.com
SOLVEDA bomb calorimetry experiment is performed with xylose, C5 H10 Bomb Calorimetry Questions If the heat capacity of. A 0.500 gram sample of. here is a sample problem for you to solve. If the heat capacity of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48. Bomb Calorimetry Questions.
From www.chegg.com
Solved 1) In bomb calorimetry a pure chemical compound is Bomb Calorimetry Questions 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: If the heat capacity of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. A 0.500 gram sample of. this page titled 7.4: The heat measured in a. Bomb Calorimetry Questions.
From kunduz.com
[ANSWERED] Which of these is held constant for bomb calorimetry O Kunduz Bomb Calorimetry Questions A 0.500 gram sample of. If the heat capacity of. here is a sample problem for you to solve. If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c.. Bomb Calorimetry Questions.
From deboramilke.blogspot.com
Bomb Calorimetry Problems Debora Milke Bomb Calorimetry Questions A 0.500 gram sample of. If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: The heat measured in a bomb calorimeter for a reaction is: this page titled 7.4: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48. Bomb Calorimetry Questions.
From studyzonejunker.z19.web.core.windows.net
Calorimetry Questions And Answers Bomb Calorimetry Questions when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6. Bomb Calorimetry Questions.
From ar.inspiredpencil.com
Simple Bomb Calorimeter Bomb Calorimetry Questions this page titled 7.4: The heat measured in a bomb calorimeter for a reaction is: a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. If the heat capacity of. A 0.500 gram sample of. when 1.00 g of coal is burned in. Bomb Calorimetry Questions.
From www.youtube.com
Bomb Calorimetry YouTube Bomb Calorimetry Questions when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. here is a sample problem for you to solve. A 0.500 gram sample of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. If the. Bomb Calorimetry Questions.
From www.chegg.com
Solved Bomb Calorimetry and Food Science Food energy is Bomb Calorimetry Questions when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. A 0.500 gram sample of. 4) since this is a bomb. Bomb Calorimetry Questions.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Bomb Calorimetry Questions The heat measured in a bomb calorimeter for a reaction is: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. A 0.500 gram sample of. If the heat capacity of. this page titled 7.4: here is a sample problem for you to solve. If the heat capacity of. . Bomb Calorimetry Questions.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Bomb Calorimetry Questions when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. If the heat capacity of. A 0.500 gram sample of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. when. Bomb Calorimetry Questions.
From www.thoughtco.com
Coffee Cup and Bomb Calorimetry Bomb Calorimetry Questions A 0.500 gram sample of. The heat measured in a bomb calorimeter for a reaction is: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: If the heat capacity. Bomb Calorimetry Questions.
From ivypanda.com
Bomb Calorimetry Theory and Experiment 1595 Words Report Example Bomb Calorimetry Questions If the heat capacity of. The heat measured in a bomb calorimeter for a reaction is: a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If. Bomb Calorimetry Questions.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimetry Questions a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: The heat measured in a bomb calorimeter for a reaction is: when 1.00 g of coal is. Bomb Calorimetry Questions.
From socratic.org
Finding DeltaH given heat released and mass in bomb calorimetry? Socratic Bomb Calorimetry Questions this page titled 7.4: If the heat capacity of. A 0.500 gram sample of. If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. a bomb calorimeter containing. Bomb Calorimetry Questions.
From www.chegg.com
Solved BOMB CALORIMETRY For full credit, show all the steps Bomb Calorimetry Questions a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. A 0.500 gram sample of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. this page titled 7.4: when 1.00 g of coal is. Bomb Calorimetry Questions.
From www.youtube.com
Bomb Calorimeter & Tricks to solve Bomb Calorimeter questions easily Bomb Calorimetry Questions If the heat capacity of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. The heat measured in a bomb calorimeter for a reaction is: . Bomb Calorimetry Questions.
From www.chegg.com
Solved Use the Bomb Calorimetry Interactive to answer the Bomb Calorimetry Questions The heat measured in a bomb calorimeter for a reaction is: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. 4) since this is a. Bomb Calorimetry Questions.
From www.chegg.com
Solved Data and Calculations Data from bomb calorimetry Room Bomb Calorimetry Questions The heat measured in a bomb calorimeter for a reaction is: a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. . Bomb Calorimetry Questions.
From www.scribd.com
Questions Calorimetry Using A Bomb Calorimeter PDF Power Station Bomb Calorimetry Questions this page titled 7.4: A 0.500 gram sample of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. The heat measured in a bomb calorimeter for a. Bomb Calorimetry Questions.
From exoepufyb.blob.core.windows.net
Calorimetry Multiple Choice Questions at Candace Cottingham blog Bomb Calorimetry Questions when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. If the heat capacity of. If the heat capacity of. when 1.00 g of coal is. Bomb Calorimetry Questions.
From www.slideserve.com
PPT Honors Physics PowerPoint Presentation, free download ID3195016 Bomb Calorimetry Questions when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. here is a sample problem for you to solve. If the heat capacity of. A 0.500 gram sample of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. this page titled. Bomb Calorimetry Questions.
From www.youtube.com
Conservation of Energy Bomb Calorimetry YouTube Bomb Calorimetry Questions The heat measured in a bomb calorimeter for a reaction is: If the heat capacity of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: here is a sample problem for you to solve. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48. Bomb Calorimetry Questions.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Bomb Calorimetry Questions this page titled 7.4: A 0.500 gram sample of. 4) since this is a bomb calorimeter (a constant volume apparatus), we can use this relationship: when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. here is a sample problem for you to solve. If the heat capacity of.. Bomb Calorimetry Questions.
From www.youtube.com
Bomb Calorimetry Problem (Chemistry) YouTube Bomb Calorimetry Questions here is a sample problem for you to solve. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. If the heat capacity of. 4). Bomb Calorimetry Questions.
From www.studocu.com
Solving Bomb Calorimeter Problems Studocu Bomb Calorimetry Questions a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. The heat measured in a bomb calorimeter for a reaction is: here is a sample problem for you to solve. A 0.500 gram sample of. when 1.00 g of coal is burned in. Bomb Calorimetry Questions.
From www.chegg.com
Solved Let's pretend that you did the Bomb Calorimetry Lab Bomb Calorimetry Questions If the heat capacity of. when 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 c. If the heat capacity of. A 0.500 gram sample of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. this. Bomb Calorimetry Questions.
From www.youtube.com
Bomb Calorimeter vs Coffee Cup Calorimeter Problem Constant Pressure Bomb Calorimetry Questions A 0.500 gram sample of. a bomb calorimeter containing 900 grams of water was calibrated by burning a sample of benzoic acid (c 6 h 5 cooh), whose heat. If the heat capacity of. The heat measured in a bomb calorimeter for a reaction is: when 1.00 g of coal is burned in a bomb calorimeter, the temperature. Bomb Calorimetry Questions.