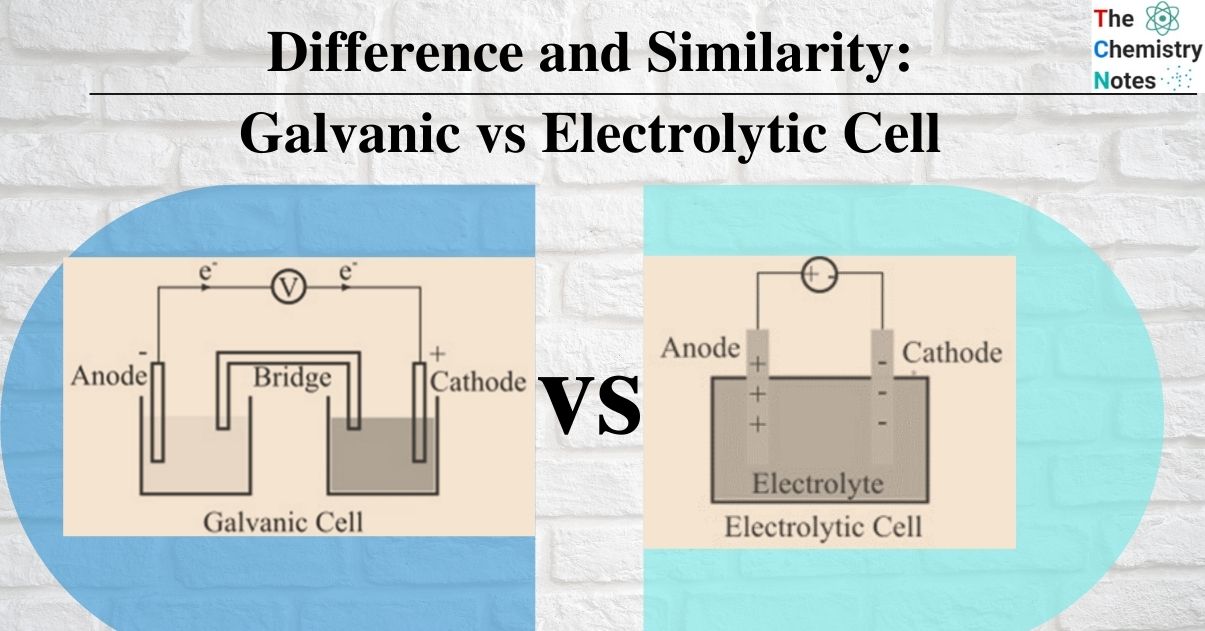
How Do Galvanic Cell Differ From Electrolytic Cell . in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous.
from thechemistrynotes.com
electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external.
Difference and Similarity Galvanic vs Electrolytic Cell
How Do Galvanic Cell Differ From Electrolytic Cell galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical.
From www.youtube.com
What is the Difference between Galvanic cell and Electrolytic cell How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. in. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell, also known as a voltaic cell,. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. . How Do Galvanic Cell Differ From Electrolytic Cell.
From thechemistrynotes.com
Difference and Similarity Galvanic vs Electrolytic Cell How Do Galvanic Cell Differ From Electrolytic Cell galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic cell, also known as a voltaic. How Do Galvanic Cell Differ From Electrolytic Cell.
From jesse-has-lozano.blogspot.com
Explain the Difference Between a Galvanic Cell and Electrolytic Cell How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic cell, also known as a voltaic. How Do Galvanic Cell Differ From Electrolytic Cell.
From employees.csbsju.edu
CHEM 123 GENERAL CHEMISTRY 1 How Do Galvanic Cell Differ From Electrolytic Cell in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. electrolytic. How Do Galvanic Cell Differ From Electrolytic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry How Do Galvanic Cell Differ From Electrolytic Cell in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.pinterest.co.uk
What are the differences between Galvanic cell and Electrolytic cell How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download How Do Galvanic Cell Differ From Electrolytic Cell in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell is an. How Do Galvanic Cell Differ From Electrolytic Cell.
From quizturbinates.z21.web.core.windows.net
How Does Galvanic Cell Work How Do Galvanic Cell Differ From Electrolytic Cell electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. galvanic cells generate electrical energy. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.youtube.com
Galvanic Cell vs Electrolytic Cell animation Electrochemical Cells How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from. How Do Galvanic Cell Differ From Electrolytic Cell.
From chemwiki.ucdavis.edu
Electrolytic Cells Chemwiki How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. . How Do Galvanic Cell Differ From Electrolytic Cell.
From www.youtube.com
GALVANIC CELL V/S COMMON ELECTROLYTIC CELL YouTube How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. electrolytic cells and galvanic cells are both types. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.youtube.com
Galvanic versus Electrolytic Cells YouTube How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic cell is an electrochemical. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.youtube.com
Galvanic Cell Vs Electrolytic Cell differences YouTube How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical. How Do Galvanic Cell Differ From Electrolytic Cell.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog How Do Galvanic Cell Differ From Electrolytic Cell electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes. How Do Galvanic Cell Differ From Electrolytic Cell.
From socratic.org
How can a galvanic cell an electrolytic cell? Socratic How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic cell, also known as. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. How Do Galvanic Cell Differ From Electrolytic Cell.
From exoyksicy.blob.core.windows.net
Distinguish Between Galvanic Cell And Electrolytic Cell at John Rowe blog How Do Galvanic Cell Differ From Electrolytic Cell electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. . How Do Galvanic Cell Differ From Electrolytic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. . How Do Galvanic Cell Differ From Electrolytic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell How Do Galvanic Cell Differ From Electrolytic Cell in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell is an. How Do Galvanic Cell Differ From Electrolytic Cell.
From courses.lumenlearning.com
Galvanic Cells Chemistry How Do Galvanic Cell Differ From Electrolytic Cell in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. electrolytic cells and galvanic cells are both types. How Do Galvanic Cell Differ From Electrolytic Cell.
From exodrddcz.blob.core.windows.net
Difference Between Galvanic And Electrolytic Cell at Brian Chambless blog How Do Galvanic Cell Differ From Electrolytic Cell electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell is an electrochemical. How Do Galvanic Cell Differ From Electrolytic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell How Do Galvanic Cell Differ From Electrolytic Cell electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. . How Do Galvanic Cell Differ From Electrolytic Cell.
From differguide.com
Difference Between Galvanic And Electrolytic Cell How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.geeksforgeeks.org
Electrochemical Cell Definition, Types, Working Principle & Uses How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. galvanic cells generate electrical energy through spontaneous redox reactions,. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.slideserve.com
PPT Galvanic and Electrolytic Cells PowerPoint Presentation, free How Do Galvanic Cell Differ From Electrolytic Cell a galvanic cell is an electrochemical cell in which spontaneous redox processes occur allowing the. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell, also known as a voltaic. How Do Galvanic Cell Differ From Electrolytic Cell.
From ar.inspiredpencil.com
Galvanic Cell Vs Electrolytic Cell How Do Galvanic Cell Differ From Electrolytic Cell in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. a galvanic cell, also known as. How Do Galvanic Cell Differ From Electrolytic Cell.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. galvanic cells generate electrical energy through spontaneous redox reactions, while electrolytic cells require an external. a galvanic cell is an electrochemical cell in which. How Do Galvanic Cell Differ From Electrolytic Cell.
From mavink.com
Differentiate Between Electrochemical Cell And Electrolytic Cell How Do Galvanic Cell Differ From Electrolytic Cell electrolytic cells and galvanic cells are both types of electrochemical cells used to convert chemical energy into electrical. a galvanic cell, also known as a voltaic cell, is an electrochemical cell that converts chemical energy into electrical energy through a spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity,. How Do Galvanic Cell Differ From Electrolytic Cell.
From quizturbinates.z21.web.core.windows.net
How Does Galvanic Cell Work How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. galvanic cells generate electrical energy through. How Do Galvanic Cell Differ From Electrolytic Cell.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic cell, also known as. How Do Galvanic Cell Differ From Electrolytic Cell.
From pediaa.com
Difference Between Electrochemical Cell and Electrolytic Cell How Do Galvanic Cell Differ From Electrolytic Cell a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electrical energy from an external source to force a reaction to occur. in a galvanic cell, spontaneous chemical reactions produce electrical energy, while in an electrolytic cell, electrical energy is. a galvanic cell, also known as. How Do Galvanic Cell Differ From Electrolytic Cell.