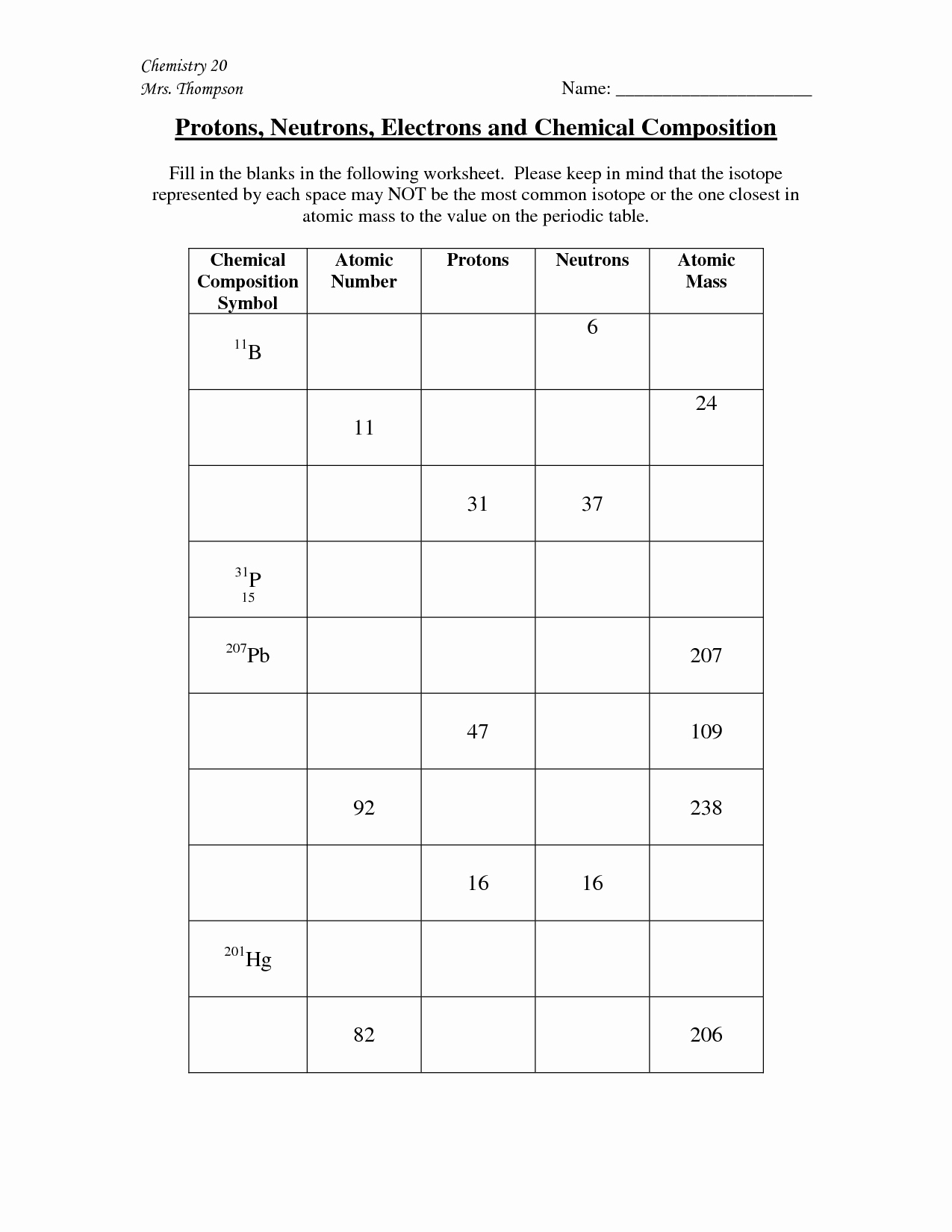
How Do Isotopes Differ Worksheet Answers . • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. All atoms want to completely fill their outermost energy shell: we can see that the difference between the isotopes is that they have different mass numbers. 2 electrons maximum in the. 8 electrons ( no more allowed) octet rule. They have the same number of protons and electrons as the element but different mass numbers and number of. Isotopes of the same element have different mass number because they have a different number of. isotopes are versions of the same element. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. answer the following questions: 3) how do different isotopes of the same element differ?
from chessmuseum.org
3) how do different isotopes of the same element differ? answer the following questions: They have the same number of protons and electrons as the element but different mass numbers and number of. 2 electrons maximum in the. 8 electrons ( no more allowed) octet rule. we can see that the difference between the isotopes is that they have different mass numbers. Isotopes of the same element have different mass number because they have a different number of. isotopes are versions of the same element. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons.
50 Isotope Practice Worksheet Answer Key
How Do Isotopes Differ Worksheet Answers All atoms want to completely fill their outermost energy shell: study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. They have the same number of protons and electrons as the element but different mass numbers and number of. All atoms want to completely fill their outermost energy shell: 2 electrons maximum in the. 3) how do different isotopes of the same element differ? answer the following questions: isotopes are versions of the same element. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. Isotopes of the same element have different mass number because they have a different number of. 8 electrons ( no more allowed) octet rule. we can see that the difference between the isotopes is that they have different mass numbers.
From www.studocu.com
Isotopes worksheet answer key PART I. Answer the questions based on How Do Isotopes Differ Worksheet Answers All atoms want to completely fill their outermost energy shell: 3) how do different isotopes of the same element differ? 8 electrons ( no more allowed) octet rule. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. • identify the composition of atoms and. How Do Isotopes Differ Worksheet Answers.
From wordworksheet.com
Isotope Practice Worksheet Answers How Do Isotopes Differ Worksheet Answers isotopes are versions of the same element. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. All atoms want to completely fill their outermost energy shell: 2 electrons maximum in the. answer the following questions: Isotopes of the same element have different mass number because they have. How Do Isotopes Differ Worksheet Answers.
From kidsworksheetfun.com
Isotopes Worksheet 1 Answer Key Kidsworksheetfun How Do Isotopes Differ Worksheet Answers Isotopes of the same element have different mass number because they have a different number of. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. isotopes are versions of the same element. 3) how do different isotopes of the same element differ? They have the. How Do Isotopes Differ Worksheet Answers.
From www.studypool.com
SOLUTION Isotopes worksheet answer key Studypool How Do Isotopes Differ Worksheet Answers we can see that the difference between the isotopes is that they have different mass numbers. Isotopes of the same element have different mass number because they have a different number of. 2 electrons maximum in the. isotopes are versions of the same element. They have the same number of protons and electrons as the element but different. How Do Isotopes Differ Worksheet Answers.
From www.madebyteachers.com
Isotope Notation Chemistry Worksheet 20 Problems With Answers Made By How Do Isotopes Differ Worksheet Answers 2 electrons maximum in the. answer the following questions: we can see that the difference between the isotopes is that they have different mass numbers. All atoms want to completely fill their outermost energy shell: study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. . How Do Isotopes Differ Worksheet Answers.
From studylib.net
Isotopes Worksheet How Do Isotopes Differ Worksheet Answers Isotopes of the same element have different mass number because they have a different number of. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. 3) how do different isotopes of the same element differ? All atoms want to completely fill their outermost energy shell: 2 electrons maximum. How Do Isotopes Differ Worksheet Answers.
From www.scribd.com
Isotopes_Worksheet_Answers (1).pdf How Do Isotopes Differ Worksheet Answers study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. 3) how do different isotopes of the same element differ? All atoms want to completely fill their outermost energy shell: • identify the composition of atoms and their isotopes in terms of the numbers of protons,. How Do Isotopes Differ Worksheet Answers.
From answerlistjurgen.z19.web.core.windows.net
Isotope Practice Worksheets With Answers How Do Isotopes Differ Worksheet Answers They have the same number of protons and electrons as the element but different mass numbers and number of. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. Isotopes of the same element have different mass number because they have a different number of. All atoms want to completely. How Do Isotopes Differ Worksheet Answers.
From www.artofit.org
Isotopes worksheet review and practice problems Artofit How Do Isotopes Differ Worksheet Answers Isotopes of the same element have different mass number because they have a different number of. They have the same number of protons and electrons as the element but different mass numbers and number of. answer the following questions: 3) how do different isotopes of the same element differ? study with quizlet and memorize flashcards containing terms. How Do Isotopes Differ Worksheet Answers.
From worksheetschoolcleve.z13.web.core.windows.net
Physical Science Isotopes Worksheet How Do Isotopes Differ Worksheet Answers • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. 2 electrons maximum in the. All atoms want to completely fill their outermost energy shell: Isotopes of the same element have different mass number because they have a different number of. answer the following questions: isotopes are versions. How Do Isotopes Differ Worksheet Answers.
From learningphallus.z13.web.core.windows.net
Practice Isotope Calculations 1 Worksheet Answers How Do Isotopes Differ Worksheet Answers Isotopes of the same element have different mass number because they have a different number of. 2 electrons maximum in the. isotopes are versions of the same element. They have the same number of protons and electrons as the element but different mass numbers and number of. • identify the composition of atoms and their isotopes in terms. How Do Isotopes Differ Worksheet Answers.
From kidsworksheetfun.com
Isotopes Worksheet 1 Answer Key Kidsworksheetfun How Do Isotopes Differ Worksheet Answers 3) how do different isotopes of the same element differ? we can see that the difference between the isotopes is that they have different mass numbers. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. Isotopes of the same element have different mass number because. How Do Isotopes Differ Worksheet Answers.
From studydblang99.z21.web.core.windows.net
Isotope Practice Worksheet Answer Key How Do Isotopes Differ Worksheet Answers answer the following questions: • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. 2 electrons maximum in the. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. 3) how do different isotopes of the. How Do Isotopes Differ Worksheet Answers.
From wordworksheet.com
Isotope Practice Worksheet Answers How Do Isotopes Differ Worksheet Answers we can see that the difference between the isotopes is that they have different mass numbers. 2 electrons maximum in the. All atoms want to completely fill their outermost energy shell: They have the same number of protons and electrons as the element but different mass numbers and number of. 3) how do different isotopes of the same. How Do Isotopes Differ Worksheet Answers.
From www.e-streetlight.com
Isotopes Worksheet Answer Key How Do Isotopes Differ Worksheet Answers 2 electrons maximum in the. we can see that the difference between the isotopes is that they have different mass numbers. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. • identify the composition of atoms and their isotopes in terms of the numbers of. How Do Isotopes Differ Worksheet Answers.
From wordworksheet.com
Atoms And Isotopes Worksheet Answers How Do Isotopes Differ Worksheet Answers we can see that the difference between the isotopes is that they have different mass numbers. They have the same number of protons and electrons as the element but different mass numbers and number of. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. 2 electrons maximum in. How Do Isotopes Differ Worksheet Answers.
From www.chemistrylearner.com
Free Printable Isotopes Worksheets How Do Isotopes Differ Worksheet Answers They have the same number of protons and electrons as the element but different mass numbers and number of. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. Isotopes of the same element have different mass number because they have a different number of. 2 electrons maximum in the.. How Do Isotopes Differ Worksheet Answers.
From www.docsity.com
Isotope Practice Worksheet Lecture notes Chemistry Docsity How Do Isotopes Differ Worksheet Answers 2 electrons maximum in the. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. 8 electrons ( no more allowed) octet rule. we can see that the difference between the isotopes is that they have different mass numbers. isotopes are versions of the same element. They. How Do Isotopes Differ Worksheet Answers.
From www.housview.com
Isotope Problems Worksheets Answers How Do Isotopes Differ Worksheet Answers 2 electrons maximum in the. isotopes are versions of the same element. answer the following questions: They have the same number of protons and electrons as the element but different mass numbers and number of. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. we can. How Do Isotopes Differ Worksheet Answers.
From www.tes.com
Isotopes worksheets and full answers Teaching Resources How Do Isotopes Differ Worksheet Answers They have the same number of protons and electrons as the element but different mass numbers and number of. 2 electrons maximum in the. All atoms want to completely fill their outermost energy shell: • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. study with quizlet and memorize. How Do Isotopes Differ Worksheet Answers.
From www.chemistrylearner.com
Free Printable Atomic Structure And Isotopes Worksheets How Do Isotopes Differ Worksheet Answers isotopes are versions of the same element. 3) how do different isotopes of the same element differ? They have the same number of protons and electrons as the element but different mass numbers and number of. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why.. How Do Isotopes Differ Worksheet Answers.
From answerlistwade123.z13.web.core.windows.net
Isotopes And Average Atomic Mass Worksheet Answers How Do Isotopes Differ Worksheet Answers we can see that the difference between the isotopes is that they have different mass numbers. Isotopes of the same element have different mass number because they have a different number of. study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. All atoms want to completely. How Do Isotopes Differ Worksheet Answers.
From www.pinterest.com
Isotope Practice Worksheet Answers Beautiful isotopes Worksheet How Do Isotopes Differ Worksheet Answers we can see that the difference between the isotopes is that they have different mass numbers. answer the following questions: Isotopes of the same element have different mass number because they have a different number of. All atoms want to completely fill their outermost energy shell: 3) how do different isotopes of the same element differ? 2. How Do Isotopes Differ Worksheet Answers.
From printablezeccolivh.z21.web.core.windows.net
Atoms Ions And Isotopes Worksheet Answer Key How Do Isotopes Differ Worksheet Answers 8 electrons ( no more allowed) octet rule. Isotopes of the same element have different mass number because they have a different number of. All atoms want to completely fill their outermost energy shell: 2 electrons maximum in the. 3) how do different isotopes of the same element differ? answer the following questions: isotopes are versions. How Do Isotopes Differ Worksheet Answers.
From studylib.net
Isotopes Worksheet How Do Isotopes Differ Worksheet Answers 3) how do different isotopes of the same element differ? Isotopes of the same element have different mass number because they have a different number of. 2 electrons maximum in the. All atoms want to completely fill their outermost energy shell: we can see that the difference between the isotopes is that they have different mass numbers. . How Do Isotopes Differ Worksheet Answers.
From www.chemistrylearner.com
Free Printable Isotopes Worksheets How Do Isotopes Differ Worksheet Answers Isotopes of the same element have different mass number because they have a different number of. All atoms want to completely fill their outermost energy shell: • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. 2 electrons maximum in the. 8 electrons ( no more allowed) octet rule.. How Do Isotopes Differ Worksheet Answers.
From www.madebyteachers.com
Isotope Notation Chemistry Worksheet 20 Problems With Answers Made By How Do Isotopes Differ Worksheet Answers Isotopes of the same element have different mass number because they have a different number of. All atoms want to completely fill their outermost energy shell: answer the following questions: study with quizlet and memorize flashcards containing terms like how do isotopes of the same element differ from each other, why. • identify the composition of atoms. How Do Isotopes Differ Worksheet Answers.
From www.studocu.com
ChemistryBridging the Gap Answer Isotope Practice Worksheet Here are How Do Isotopes Differ Worksheet Answers isotopes are versions of the same element. answer the following questions: Isotopes of the same element have different mass number because they have a different number of. They have the same number of protons and electrons as the element but different mass numbers and number of. All atoms want to completely fill their outermost energy shell: 3). How Do Isotopes Differ Worksheet Answers.
From martindxmguide.blogspot.com
32 Isotope Worksheet Answer Key support worksheet How Do Isotopes Differ Worksheet Answers 8 electrons ( no more allowed) octet rule. 2 electrons maximum in the. isotopes are versions of the same element. answer the following questions: They have the same number of protons and electrons as the element but different mass numbers and number of. Isotopes of the same element have different mass number because they have a different. How Do Isotopes Differ Worksheet Answers.
From www.onlineworksheet.my.id
Isotope Practice Worksheet Answer Key Onlineworksheet.my.id How Do Isotopes Differ Worksheet Answers They have the same number of protons and electrons as the element but different mass numbers and number of. 8 electrons ( no more allowed) octet rule. answer the following questions: • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. study with quizlet and memorize flashcards. How Do Isotopes Differ Worksheet Answers.
From www.studypool.com
SOLUTION Isotope worksheet answer key Studypool How Do Isotopes Differ Worksheet Answers Isotopes of the same element have different mass number because they have a different number of. They have the same number of protons and electrons as the element but different mass numbers and number of. answer the following questions: All atoms want to completely fill their outermost energy shell: 3) how do different isotopes of the same element. How Do Isotopes Differ Worksheet Answers.
From www.englishworksheet.my.id
Isotope Practice Worksheet Answers Englishworksheet.my.id How Do Isotopes Differ Worksheet Answers we can see that the difference between the isotopes is that they have different mass numbers. 8 electrons ( no more allowed) octet rule. answer the following questions: • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. study with quizlet and memorize flashcards containing terms. How Do Isotopes Differ Worksheet Answers.
From chessmuseum.org
50 Isotope Practice Worksheet Answer Key How Do Isotopes Differ Worksheet Answers 8 electrons ( no more allowed) octet rule. answer the following questions: 2 electrons maximum in the. Isotopes of the same element have different mass number because they have a different number of. • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. we can see that. How Do Isotopes Differ Worksheet Answers.
From www.chemistrylearner.com
Free Printable Isotopes Worksheets How Do Isotopes Differ Worksheet Answers • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. answer the following questions: 8 electrons ( no more allowed) octet rule. we can see that the difference between the isotopes is that they have different mass numbers. 2 electrons maximum in the. They have the same. How Do Isotopes Differ Worksheet Answers.
From answermediabrandt.z19.web.core.windows.net
Isotopes Worksheets With Answers How Do Isotopes Differ Worksheet Answers They have the same number of protons and electrons as the element but different mass numbers and number of. 3) how do different isotopes of the same element differ? • identify the composition of atoms and their isotopes in terms of the numbers of protons, neutrons, and electrons. Isotopes of the same element have different mass number because. How Do Isotopes Differ Worksheet Answers.