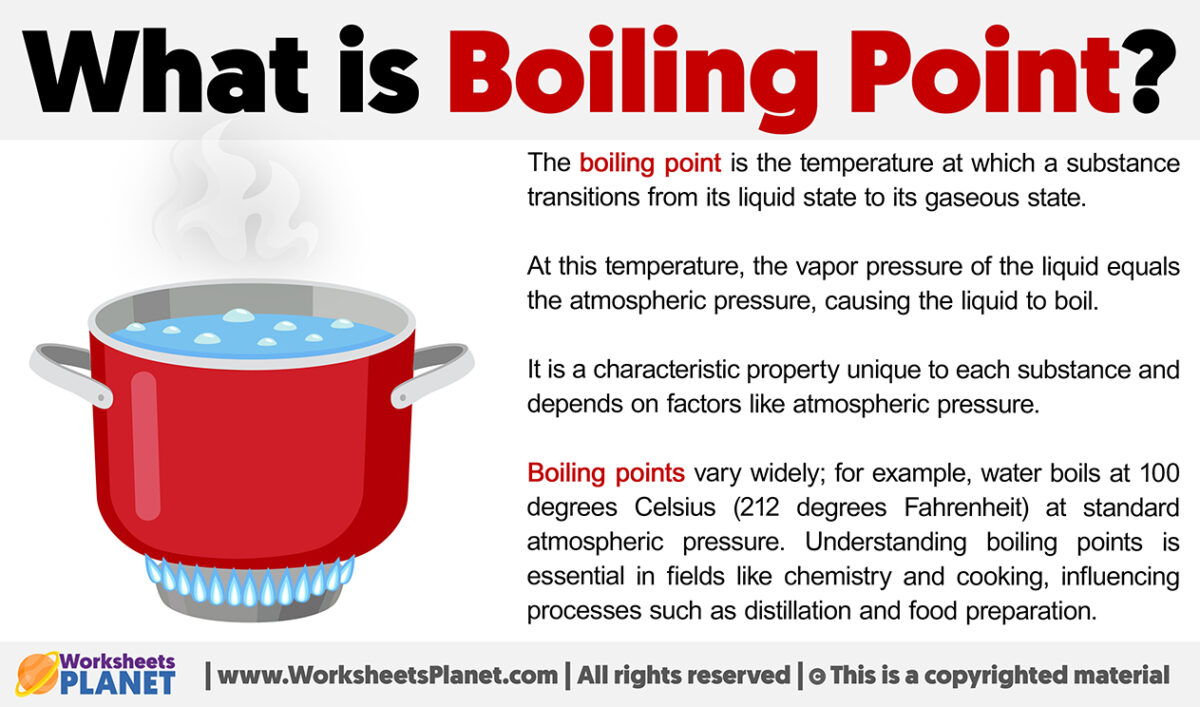
What Is A Normal Boiling Point Water . the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. This is the boiling point which is usually quoted in chemical literature. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. Not everyone lives at sea level, though. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. The normal boiling point is the temperature. the boiling point of a liquid varies according to the applied pressure; However, the value is not a constant. The standard boiling point of water is 99.61 °c at 1 bar of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level).
from www.worksheetsplanet.com
the boiling point is the temperature at which a liquid substance changes to its gaseous phase. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. the boiling point of a liquid varies according to the applied pressure; However, the value is not a constant. The normal boiling point is the temperature. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The standard boiling point of water is 99.61 °c at 1 bar of pressure. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). This is the boiling point which is usually quoted in chemical literature. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure.
What is Boiling Point
What Is A Normal Boiling Point Water The normal boiling point is the temperature. The standard boiling point of water is 99.61 °c at 1 bar of pressure. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the boiling point of a liquid varies according to the applied pressure; The normal boiling point is the temperature. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. Not everyone lives at sea level, though. This is the boiling point which is usually quoted in chemical literature. However, the value is not a constant.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff What Is A Normal Boiling Point Water the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. The standard boiling point of water is 99.61 °c at 1 bar of pressure. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. Not everyone lives at. What Is A Normal Boiling Point Water.
From manuallistcoverslip.z1.web.core.windows.net
Normal Boiling Point On Phase Diagram What Is A Normal Boiling Point Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. However, the value is not a constant. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. normal boiling point is the temperature at which a liquid. What Is A Normal Boiling Point Water.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Is A Normal Boiling Point Water if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. The standard boiling point of water is 99.61 °c at 1 bar of pressure.. What Is A Normal Boiling Point Water.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Is A Normal Boiling Point Water Not everyone lives at sea level, though. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. The standard boiling point of water is 99.61 °c at 1 bar of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. What Is A Normal Boiling Point Water.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download What Is A Normal Boiling Point Water Not everyone lives at sea level, though. However, the value is not a constant. This is the boiling point which is usually quoted in chemical literature. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). the boiling. What Is A Normal Boiling Point Water.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Normal Boiling Point Water However, the value is not a constant. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. the boiling point is the temperature. What Is A Normal Boiling Point Water.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Is A Normal Boiling Point Water normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. This is the boiling point which is usually quoted in chemical literature. Not everyone lives at sea level,. What Is A Normal Boiling Point Water.
From www.thoughtco.com
Normal Boiling Point Definition (Chemistry) What Is A Normal Boiling Point Water This is the boiling point which is usually quoted in chemical literature. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. However, the. What Is A Normal Boiling Point Water.
From askfilo.com
Difference of normal boiling point and standard boiling point of water is.. What Is A Normal Boiling Point Water Not everyone lives at sea level, though. the boiling point of a liquid varies according to the applied pressure; The standard boiling point of water is 99.61 °c at 1 bar of pressure. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. This. What Is A Normal Boiling Point Water.
From schematicdiagramtelium.z13.web.core.windows.net
How To Calculate The Normal Boiling Point What Is A Normal Boiling Point Water if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. This is the boiling point which is usually quoted in chemical literature. if you want a quick and simple answer, you can say that the boiling point of water is. What Is A Normal Boiling Point Water.
From dbcarnahanicosahedra.z21.web.core.windows.net
Normal Boiling Point On Phase Diagram What Is A Normal Boiling Point Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. This is the boiling point which is usually quoted in chemical literature. The standard boiling point of water is 99.61 °c at 1 bar of pressure. if you want a quick and simple answer, you can say that the boiling point of. What Is A Normal Boiling Point Water.
From guidemanualeruptivity.z14.web.core.windows.net
How To Determine Normal Boiling Point What Is A Normal Boiling Point Water This is the boiling point which is usually quoted in chemical literature. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The standard boiling point of water is 99.61 °c at 1 bar of pressure. normal boiling point is. What Is A Normal Boiling Point Water.
From www.coursehero.com
[Solved] The table below shows the normal boiling points of several What Is A Normal Boiling Point Water This is the boiling point which is usually quoted in chemical literature. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). The normal boiling point is the temperature. However, the value is not a constant. normal boiling. What Is A Normal Boiling Point Water.
From wirepartfrontwards.z14.web.core.windows.net
Phase Diagram Normal Boiling Point What Is A Normal Boiling Point Water This is the boiling point which is usually quoted in chemical literature. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. However, the value is not a constant. The normal boiling point is the temperature. The standard boiling point of water is 99.61 °c at 1 bar of pressure. if this. What Is A Normal Boiling Point Water.
From wiringliststaphyloma.z14.web.core.windows.net
How To Calculate The Normal Boiling Point What Is A Normal Boiling Point Water if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level).. What Is A Normal Boiling Point Water.
From www.animalia-life.club
Boiling Point Of Water Examples What Is A Normal Boiling Point Water However, the value is not a constant. Not everyone lives at sea level, though. This is the boiling point which is usually quoted in chemical literature. the boiling point of a liquid varies according to the applied pressure; the boiling point is the temperature at which a liquid substance changes to its gaseous phase. the standard boiling. What Is A Normal Boiling Point Water.
From differencebtw.com
Normal Boiling Point vs. Standard Boiling Point Know the Difference What Is A Normal Boiling Point Water However, the value is not a constant. Not everyone lives at sea level, though. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). the boiling point of a liquid varies according to the applied pressure; The normal. What Is A Normal Boiling Point Water.
From wirepartentangling.z21.web.core.windows.net
Boiling Point Of Water Diagram What Is A Normal Boiling Point Water The normal boiling point is the temperature. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). This is the boiling point which is usually quoted in chemical literature. the boiling point of a liquid varies according to. What Is A Normal Boiling Point Water.
From drivenheisenberg.blogspot.com
The Normal Boiling Point For The Substance In The Phase Diagram Below What Is A Normal Boiling Point Water Not everyone lives at sea level, though. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). the boiling point of. What Is A Normal Boiling Point Water.
From mavink.com
Phase Diagram Normal Boiling Point What Is A Normal Boiling Point Water However, the value is not a constant. This is the boiling point which is usually quoted in chemical literature. The normal boiling point is the temperature. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. Not everyone lives at sea level, though. if you want a quick and simple answer, you. What Is A Normal Boiling Point Water.
From schematiclistsalem123.z22.web.core.windows.net
Phase Diagram Normal Boiling Point What Is A Normal Boiling Point Water This is the boiling point which is usually quoted in chemical literature. Not everyone lives at sea level, though. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. the boiling point of a liquid varies according to the applied pressure; the standard boiling point, as defined by the iupac in. What Is A Normal Boiling Point Water.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is A Normal Boiling Point Water The normal boiling point is the temperature. if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). Not everyone lives at sea level, though. This is the boiling point which is usually quoted in chemical literature. normal boiling. What Is A Normal Boiling Point Water.
From www.toppr.com
The normal boiling point of water is 373 K . V What Is A Normal Boiling Point Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. However, the value is not a constant. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. Not everyone lives at sea level, though. the boiling point of a liquid varies according to the applied. What Is A Normal Boiling Point Water.
From fyoyitjlg.blob.core.windows.net
What Is Normal Boiling Point And Standard at Mel Belcher blog What Is A Normal Boiling Point Water The normal boiling point is the temperature. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. Not everyone lives at sea level, though. The standard boiling point of water is 99.61 °c at 1 bar of pressure. if you want a quick and simple answer, you can say that the boiling. What Is A Normal Boiling Point Water.
From exyshludx.blob.core.windows.net
What Are The Boiling And Freezing Points Of Water How Could You Observe What Is A Normal Boiling Point Water However, the value is not a constant. The standard boiling point of water is 99.61 °c at 1 bar of pressure. This is the boiling point which is usually quoted in chemical literature. the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. Not everyone. What Is A Normal Boiling Point Water.
From guidemanualeruptivity.z14.web.core.windows.net
How To Determine Normal Boiling Point What Is A Normal Boiling Point Water the boiling point of a liquid varies according to the applied pressure; The standard boiling point of water is 99.61 °c at 1 bar of pressure. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of. What Is A Normal Boiling Point Water.
From askfilo.com
The normal boiling point of pure water is 373.15 K. Calculate the boiling.. What Is A Normal Boiling Point Water normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. However, the value is not a constant. the boiling point is the temperature. What Is A Normal Boiling Point Water.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Is A Normal Boiling Point Water the boiling point of a liquid varies according to the applied pressure; This is the boiling point which is usually quoted in chemical literature. normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which. What Is A Normal Boiling Point Water.
From mavink.com
Melting And Boiling Point Chart What Is A Normal Boiling Point Water Not everyone lives at sea level, though. the boiling point is the temperature at which a liquid substance changes to its gaseous phase. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. The normal boiling point is the temperature.. What Is A Normal Boiling Point Water.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Is A Normal Boiling Point Water the standard boiling point, as defined by the iupac in 1982, is the temperature at which boiling occurs when the pressure is 1 bar. the boiling point of a liquid varies according to the applied pressure; the boiling point is the temperature at which a liquid substance changes to its gaseous phase. normal boiling point is. What Is A Normal Boiling Point Water.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is A Normal Boiling Point Water This is the boiling point which is usually quoted in chemical literature. the boiling point of a liquid varies according to the applied pressure; if you want a quick and simple answer, you can say that the boiling point of water is 100 °c or 212 °f at 1 atmosphere of pressure (sea level). the boiling point. What Is A Normal Boiling Point Water.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Is A Normal Boiling Point Water the boiling point is the temperature at which a liquid substance changes to its gaseous phase. Not everyone lives at sea level, though. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. if you want a quick and. What Is A Normal Boiling Point Water.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Is A Normal Boiling Point Water This is the boiling point which is usually quoted in chemical literature. However, the value is not a constant. if this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is referred to as its normal boiling point. if you want a quick and simple answer, you can say. What Is A Normal Boiling Point Water.
From www.worksheetsplanet.com
What is Boiling Point What Is A Normal Boiling Point Water normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. This is the boiling point which is usually quoted in chemical literature. The standard boiling point of water is 99.61 °c at 1 bar of pressure. the standard boiling point, as defined by the iupac in 1982, is the temperature at which. What Is A Normal Boiling Point Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is A Normal Boiling Point Water the boiling point of a liquid varies according to the applied pressure; normal boiling point is the temperature at which a liquid boils at 1 atmosphere of pressure. The normal boiling point is the temperature. Not everyone lives at sea level, though. This is the boiling point which is usually quoted in chemical literature. the standard boiling. What Is A Normal Boiling Point Water.